User login
This review focuses on several elements in the National Asthma Education and Prevention Program’s new guidelines, the third Expert Panel Report (EPR3),1 that differ substantially from those in EPR2,2 issued in 1997 and updated in 2002.3 These differences in approach to the management of asthma described in EPR3 offer a clear potential for reducing the gap between optimal asthma care outcomes as described in guidelines and normative asthma care outcomes in the “real world.”
GREATER EMPHASIS ON CONTROL
The EPR2 guidelines2 recommended that asthma management be carried out in an algorithmic manner. Patients were classified into four severity categories: mild intermittent, mild persistent, moderate persistent, and severe persistent asthma, based on assessment of the level of symptoms (day/night), reliance on “reliever” medication, and lung function at the time of presentation. Pharmacologic management was then assigned according to each respective categorization in an evidence-based fashion.
In an ideal world, this would result in patients with asthma receiving appropriate pharmacotherapeutic agents associated with favorable asthma care outcomes, which were also advantageous from both cost- and risk-benefit standpoints. In the real world, however, this paradigm was flawed, as it relied on accurate categorization of patients in order for pharmacotherapy to be prescribed appropriately. Both providers and patients are prone to underestimate asthma severity,4,5 and for this reason many patients managed on the basis of this paradigm were undertreated.
A new paradigm, based on the assessment of asthma control, has been encouraged in the EPR3 guidelines.1
Severity and control are not synonymous
More than a decade ago, Cockroft and Swystun6 pointed out that asthma control (or lack thereof) is often used inappropriately to define asthma severity: ie, well-controlled asthma is seen as synonymous with mild asthma, and poorly controlled asthma with severe asthma.
Asthma severity can be defined as the intrinsic intensity of the disease process, while asthma control is the degree to which the manifestations of asthma are minimized. Asthma severity is clearly a determinant of asthma control, but its impact is affected by a variety of factors, including but not limited to:
- Whether appropriate medication is prescribed
- Patterns of therapeutic adherence
- The degree to which recommended measures for avoiding for clinically relevant aeroallergens are pursued.
Health care utilization, including hospitalizations and emergency department visits, correlates more closely with asthma control than with asthma severity.7–9 Indeed, a patient with severe persistent asthma who is treated appropriately with multiple “controller” medications and who takes his or her medications and avoids allergens as directed can achieve well-controlled or totally controlled asthma, and is not likely to require hospitalization or emergency department management, to miss school or work, or to experience nocturnal awakening or limitation in routine activities due to asthma. This patient has severe persistent asthma that is well controlled.
In contrast, a patient with mild or moderate persistent asthma who does not receive appropriate instructions for avoiding allergens or taking controller medication regularly or who is poorly adherent will likely have poor asthma control. This patient is more likely to require hospitalization or emergency department management, to miss school or work, and to experience nocturnal awakening or limitation in routine activities due to asthma. This patient has mild persistent asthma that is poorly controlled.
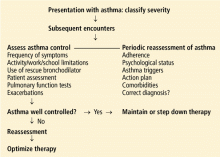
Assess asthma severity in the first visit, and control in subsequent visits
How to assess severity
How to measure control
For all patients with asthma, regardless of severity, the goal is the same: to achieve control by reducing both impairment and risk. Asthma is classified as well controlled, not well controlled, or poorly controlled (Table 2).1
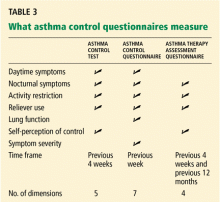
Validated tests are available to assess control
Serial testing as a quality indicator
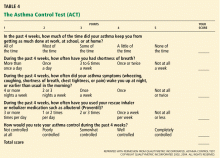
Serial ACT scores give an objective measure of the degree to which the goals of management1 are being achieved, and in so doing can encourage optimal outcomes.14
Another use of these tests is to document whether asthma control improves over time when patients receive care from a particular physician or group. This use may become increasingly important in view of efforts underway to implement a pay-for-performance model for asthma care, in which providers will be financially rewarded for improved patient care outcomes and adherence to standards of practice based on Health Plan Employer Data and Information Set measures.15
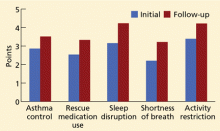
We have used the ACT in the Section of Allergy/Immunology at Cleveland Clinic for 3 years on a routine basis. All patients with asthma being seen either for the first time or as follow-up complete the ACT, which has been entered in a flow sheet in our electronic medical record, at the same time they undergo spirometry. We have shown that care in the Section of Allergy/Immunology is associated with improvement in asthma control over time, in patients who have completed serial ACT measurements at initial visits and at follow-up visits (Figure 2).
Objective measurement of lung function is also important
Serial monitoring of lung function at every patient visit with spirometry is also important, as some patients may be “poor perceivers,”16 ie, they may have little or no subjective awareness of moderate or even severe ventilatory impairment. A number of studies17,18 support the contention that symptoms and lung function are separate and independent dimensions of asthma control, and that both of them need to be assessed.
Responding to changes in control
THE STEP 3 CONTROVERSY
Salmeterol Multicenter Asthma Research Trial
In the Salmeterol Multicenter Asthma Research Trial (SMART), patients randomized to the long-acting beta agonist (LABA) salmeterol (Serevent)—particularly African Americans—had a statistically significant increase in the risk of untoward asthma care outcomes.20
SMART was launched in 1996. Patients were randomized in a double-blind fashion to receive either salmeterol 42 μg twice a day or placebo in addition to their usual asthma therapy for 28 weeks. The rate of the primary outcome (respiratory-related deaths or life-threatening experiences) was not significantly different with salmeterol than with placebo (relative risk [RR] = 1.40, 95% confidence interval [CI] 0.91–2.14). However, in 2003, the study was halted prematurely because of difficulty enrolling the targeted number of 60,000 patients, and an interim analysis that revealed significantly higher rates of secondary outcomes in subjects randomized to salmeterol. Compared with the placebo group, the salmeterol group had significantly higher rates of respiratory-related deaths (RR 2.16, 95% CI 1.06–4.41), asthma-related deaths (RR = 4.37, 95% CI = 1.25–15.34), and combined asthma-related deaths or life-threatening experiences (RR = 1.71, 95% CI 1.01–2.89). There were 13 asthma-related deaths and 37 combined asthma-related deaths or life-threatening experiences in the salmeterol group, compared with 3 and 22, respectively, in the placebo group. Of the 16 asthma deaths in the study, 13 (81%) occurred in the initial phase of SMART, when patients were recruited via print, radio, and television advertising; afterward, patients were recruited directly by investigators.
Statistically significant differences in outcomes occurred primarily in African Americans. African Americans who received salmeterol had higher rates of respiratory death or life-threatening experiences (RR = 4.10, 95% CI 1.54–10.90), the primary end point for the study, as well as higher rates of combined asthma-related deaths or life-threatening experiences (RR = 10.46, 95% CI 1.34–81.58), a secondary end point. No statistically significant differences were observed in white patients randomized to salmeterol with respect to the primary end point (RR = 1.05, 95% = 0.62–1.76); the secondary end point of combined asthma-related deaths or life-threatening experiences (RR = 1.08, 95% CI 0.55–2.14); or other end points.
Medication exposures were not tracked during the study, and allocation to inhaled corticosteroids combined with salmeterol was not randomized, so the effect of concomitant inhaled corticosteroid use cannot be determined from these data.
As a result of SMART, medications that contain either of the two LABAs, salmeterol or formoterol (Foradil), carry a black-box warning.
LABAs: Risks and benefits
Two studies21,22 have suggested that asthmatic patients who are homozygous for Arg/Arg at codon 16 of the beta-2 adrenergic receptor are predisposed to untoward asthma outcomes with regular exposure to LABAs. However, other data23–25 do not support the contention that B16 Arg/Arg patients experience adverse asthma outcomes with LABA exposure. In two recently published studies, no difference in rates of exacerbations, severe exacerbations, lung function, frequency of reliance on SABA, or nocturnal awakenings was observed in patients receiving formoterol combined with budesonide24 or salmeterol combined with fluticasone25 according to genotype. A prospective study26 also found no statistically significant difference in exacerbation rates according to beta adrenergic receptor genotype in individuals randomized to LABA monotherapy, or LABA combined with inhaled corticosteroids.
The updated EPR2 asthma guidelines,3 published in November 2002, stipulated that LABAs were the preferred controller agent to “add on” to low-dose inhaled corticosteroids for patients with moderate persistent asthma, and that the combination of low-dose inhaled corticosteroids and LABA was associated with superior outcomes: reduction of symptoms, including nocturnal awakening, increase in lung function, improvement in health-related quality of life, decreased use of “rescue” medication, and reduced rate of exacerbations and severe exacerbations, compared with higher-dose inhaled corticosteroid monotherapy. This management recommendation was categorized as level A, on the basis of data from multiple randomized, controlled, double-blinded trials.27–29 Additional evidence14,30 and data from two meta-analyses31,32 have provided further support for this recommendation, while no evidence linking LABA exposure to risk for fatal or near-fatal asthma has been found in cohort or case-control studies.33–38
Based on safety concerns, the EPR3 guidelines1 recommend that medium-dose inhaled corticosteroids be regarded as equivalent to adding LABAs to low-dose inhaled corticosteroids, and state: “the established, beneficial effects of LABA for the great majority of patients whose asthma is not well controlled with [inhaled corticosteroids] alone should be weighed against the increased risk for severe exacerbations, although uncommon, associated with daily use of LABA.”1
There is currently an honest difference of opinion39,40 among asthma specialists as to how this management recommendation for moderate persistent asthma—now depicted at “step 3” in the EPR3 guidelines (Table 4)—should be implemented. The LABA controversy was reviewed previously in the Cleveland Clinic Journal of Medicine.41
THE ROLE OF OMALIZUMAB: WEIGHING COST VS BENEFIT
The 2002 update to the EPR2 guidelines3 was issued before omalizumab (Xolair) was approved in June 2003.
Patients with severe persistent asthma are categorized in steps 5 or 6 in the EPR3 guidelines (Table 5).1 Preferred management for these patients includes inhaled corticosteroids in high doses combined with long-acting beta agonists and, for step 6 patients, oral corticosteroids.
Omalizumab was approved for management of patients with moderate or severe persistent asthma who are not achieving the goals of asthma management on inhaled corticosteroids, who exhibit a wheal-flare reaction to a perennial allergen, and whose immunoglobulin E (IgE) level is in the range of 30 to 700 IU/mL.42 Omalizumab dosing is based on the serum IgE level and on body weight.
Omalizumab, an anti-IgE monoclonal antibody
Omalizumab is a recombinant, humanized, monoclonal anti-IgE antibody that binds to IgE at the same Fc site as the high-affinity IgE receptor. Its primary mechanism of action is the binding of free IgE in the circulation, forming biologically inert, small complexes that do not activate complement and are cleared by the reticuloendothelial system.42 Its secondary mechanism of action entails a reduction in the number of high-affinity receptors on basophils, from approximately 220,000 to 8,300 receptors per cell. The latter effect was associated with a 90% reduction in histamine release from basophils in response to ex vivo challenge with dust mite allergen.43
Benefit in randomized trials
Omalizumab has been associated with statistically and clinically significant benefit in randomized, double-blind, placebo-controlled trials.44,45
Humbert et al46 randomized 419 patients whose asthma was not adequately controlled on high-dose inhaled corticosteroids and long-acting beta agonists, who were 12 to 75 years old, with reduced lung function and a history of recent asthma exacerbation, to treatment with omalizumab or placebo. Omalizumab was associated with a statistically significant reduction in the rate of asthma exacerbations and severe asthma exacerbations, as well as statistically significant improvements in asthma-related quality of life, morning peak expiratory flow rate, and asthma symptom scores.
These data support the recommendation in EPR3 to consider a trial of omalizumab in properly selected patients with severe, persistent allergic asthma.
Omalizumab is cost-beneficial in properly selected patients
The current wholesale acquisition cost of omalizumab is $532 for one 150-mg vial (David Zito, personal communication). The cost of treatment varies based on body weight and IgE level but may range from a wholesale cost of $6,388 to $38,326 per year.
However, as asthma severity increases, both direct and indirect medical expenditures increase substantially.47,48 Annual costs are approximately four times higher for severe asthma compared with mild asthma49; not only are treatment and exacerbation costs higher, but indirect costs are also disproportionately greater. Annual costs for severe asthma are significantly greater if the disease is inadequately controlled.50 For these reasons, an intervention that leads to improved outcomes for severe, poorly controlled asthma carries the potential for the greatest cost-utility for society, as it can lower direct costs by reducing the frequency and severity of exacerbations, in addition to reducing indirect medical expenditures on the basis of increased productivity and fewer days of missed work or school. The cost of omalizumab in quality-adjusted life years compares favorably with that of biologicals used in managing rheumatoid arthritis, Crohn disease, and multiple sclerosis.50
Adverse effects of omalizumab
In pivotal trials,43,44 omalizumab was associated with a substantial rate of local reactions. The rate of anaphylaxis was slightly less than 1 in 1,000, and this has been confirmed by surveillance data recorded since approval of the drug in 2003. Based on the observed risk of anaphylaxis, in July 2007, the US Food and Drug Administration added a black-box warning to the omalizumab label and stipulated that a medication guide should be provided for patients.51 The warning indicates that health care providers administering omalizumab should be prepared to manage anaphylaxis and that patients should be closely observed for an appropriate period after omalizumab administration.
The package insert also describes a numerical, but not statistically significant, increase in the rate of malignancy in patients receiving omalizumab.42 Malignancy developed in 0.5% of patients receiving omalizumab, compared with 0.2% of patients who received placebo. Because these malignancies were diagnosed over a shorter period than the time required for oncogenesis (ie, 6 months in 60% of cases), and because a heterogeneous variety of tumors was observed, there is reason to doubt these tumors were causally associated with omalizumab.
Postmarketing surveillance studies are in progress that will provide more definitive data on the potential relationship between malignancy and omalizumab exposure.
Omalizumab: Guideline recommendations
The EPR3 guidelines1 state that omalizumab is the only adjunctive therapy to demonstrate efficacy when added to high-dose inhaled corticosteroids plus long-acting beta agonists in patients with severe, persistent, allergic asthma and that evidence does not support use of the following agents, which in some cases are approved for managing other conditions and have been advocated for management of severe, refractory asthma: methotrexate, soluble interleukin (IL)-4 receptor, anti-IL-5, anti-IL-12, cyclosporine A, intravenous immune globulin, gold, troleandomycin, and colchicine. The data supporting use of macrolides were characterized as “encouraging but insufficient to support a recommendation.”
The strength of evidence for the use of omalizumab for patients in steps 5 and 6 who fulfill the criteria for its use (see above) was classified in the EPR3 guidelines1 as category B. The guidelines also say that omalizumab may be considered for adjunctive therapy in properly selected patients in step 4, as a means to avoid higher doses of inhaled corticosteroids, but that additional studies are needed to establish its utility for such patients. This recommendation was classified as category D because of the lack of published comparator trials.
ALLERGEN IMMUNOTHERAPY FOR PATIENTS WITH ASTHMA
Many patients with asthma have clinically relevant, IgE-mediated (allergic) potential to inhaled allergens.1 For patients with persistent asthma (steps 2–6 in Table 5), allergic reactions can contribute to airway inflammation, provoke symptoms, and lead to more use of medications. For this reason, identification and management of clinically relevant allergy merits consideration.52
The EPR3 guidelines1 recommend considering allergen immunotherapy for patients with mild or moderate persistent asthma (steps 2–4) who have a clinically relevant component of allergy to inhaled substances.
Changing the immune response
Allergen immunotherapy entails the incremental administration of inhalant allergens by subcutaneous injection for the purpose of inducing immune system changes in the host response. The goal of immunotherapy is to protect against allergic reactions that can be expected to occur with ongoing exposure to clinically relevant allergens.53
The immunologic changes that develop with allergen immunotherapy are complex.53,54 Successful immunotherapy results in generation of a population of CD4+/CD25+ T lymphocytes producing IL-10, transforming growth factor beta, or both. Allergen immunotherapy has been shown to block the immediate- and late-phase allergic response; to decrease recruitment of mast cells, basophils, and eosinophils on provocation or natural exposure to allergens in the skin, nose, eye, and bronchial mucosa; to blunt the seasonal rise in specific IgE; and to suppress late-phase inflammatory responses in the skin and respiratory tract. However, the efficacy of immunotherapy in relation to these immunologic changes is not completely understood.54
Many patients need skin testing
Allergen immunotherapy may be considered for patients with asthma for whom a clear relationship exists between symptoms and exposure to an allergen to which the patient is sensitive.53 Because it is often not possible to determine whether a patient is sensitive to a perennial indoor allergen (eg, dust mite) on the basis of the medical history alone,55 many patients with asthma benefit from immediate hypersensitivity skin testing to objectively assess or rule out allergy to common inhalants. In certain situations, in vitro testing may be performed, but skin testing has a higher negative predictive value and is recommended as a better screening test.56
Benefits of allergen immunotherapy
Numerous randomized, double-blind, placebo-controlled trials have shown that allergen immunotherapy is associated with benefit for reducing symptoms and medication reliance.57–63
A meta-analysis of 75 randomized, placebo-controlled studies confirmed the effectiveness of immunotherapy in asthma, with a significant reduction in asthma symptoms and medication use and with improvement in bronchial hyperreactivity.64 This meta-analysis included 36 trials of dust mite allergen, 20 of pollen, and 10 of animal dander. Immunotherapy is efficacious for pollen, mold, dust mite, cockroach, and animal allergens; however, its effectiveness is more established for dust mite, animal dander, and pollen allergens, as fewer studies have been published demonstrating efficacy using mold and cockroach allergens.53
In addition, several studies have found that children with allergic rhinitis who receive allergen immunotherapy are significantly less likely to develop asthma.65–67 Immunotherapy has also been associated with a statistically significant reduction in future sensitization to other aeroallergens.68,69
Risk of systemic reaction from allergen immunotherapy
The decision to begin allergen immunotherapy should be individualized on the basis of symptom severity, relative benefit compared with drug therapy, and whether comorbid conditions such as cardiovascular disease or beta-blocker exposure are present. These comorbid conditions are associated with heightened risk of (more serious) anaphylaxis—the major hazard of allergen immunotherapy.70 Systemic reactions during allergen immunotherapy occur at a rate of approximately 3 to 5 per 1,000 injections; for this reason, allergen immunotherapy should only be administered in a medical facility where personnel, supplies, and equipment are available to treat anaphylaxis.5
- National Heart, Lung, and Blood institute, National Asthma education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. www.nhlbi.nih.gov/guidelines/asthma. Accessed 8/7/08.
- Expert Panel Report 2: Guidelines for the diagnosis and management of asthma. U.S. Department of Health and Human Services. Publication No. 97-4051; 1997.
- Expert Panel Report: Guidelines for the diagnosis and management of asthma. Update on Selected Topics—2002. J Allergy Clin Immunol 2002; 110:S141–S207.
- FitzGerald JM, Boulet LP, McIvor RA, Zimmerman S, Chapman KR. Asthma control in Canada remains suboptimal: the Reality of Asthma Control (TRAC) study. Can Respir J 2006; 13:253–259.
- Braganza S, Sharif I, Ozuah P. Documenting asthma severity: do we get it right? J Asthma 2003; 40:661–665.
- Cockcroft DW, Swystun VA. Asthma control versus asthma severity. J Allergy Clin Immunol 1996; 98:1016–1018.
- Peters SP, Jones CA, Haselkorn T, Mink DR, Valacer DJ, Weiss ST. Real-world Evaluation of Asthma Control and Treatment (REACT): findings from a national Web-based survey. J Allergy Clin Immunol. 2007; 119:1454–1461.
- Osborne ML, Vollmer WM, Pedula KL, Wilkins J, Buist AS, O’Hollaren M. Lack of correlation of symptoms with specialist-assessed long-term asthma severity. Chest 1999; 115:85–91.
- Li JT, Oppenheimer J, Bernstein IL, et al. Attaining optimal asthma control: a practice parameter. J Allergy Clin Immunol 2005; 116:S3–S11.
- Nathan RA, Sorkness C, Kosinski M, et al. Development of the Asthma Control Test: a survey for assessing asthma control. J Allergy Clin Immunol 2004; 113:59–65.
- Schatz M, Zeiger RS, Drane A, et al. Reliability and predictive validity of the Asthma Control Test administered by telephone calls using speech recognition technology. J Allergy Clin Immunol 2007; 119:336–343.
- Peters D, Chen C, Markson LE, Allen-Ramey FC, Vollmer WM. Using an asthma control questionnaire and administrative data to predict healthcare utilization. Chest 2006; 129:918–924.
- Schatz M, Sorkness C, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006; 117:549–556.
- Bateman E, Boushey H, Bousquet J, et al. Can guideline-defined asthma control be achieved? Am J Respir Crit Care Med 2004; 170:836–844.
- Davies TJ, Bunn WB, Fromer L, Gelfand EW, Colice GL. A focus on the asthma HEDIS measure and its implications for clinical practice. Manag Care Interface 2006; 19:29–36.
- Rubinfeld AR, Pain MC. Perception of asthma. Lancet 1976; 1:882–884.
- Teeter J, Bleecker E. Relationship between airway obstruction and respiratory symptoms in adult asthmatics. Chest 1998; 113:272–277.
- Shingo S, Zhang J, Reiss T. Correlation of airway obstruction and patient reported endpoints in clinical studies. Eur Resp J 2001; 17:220–224.
- Juniper EF, Bousquet J, Abetz L, Bateman ED; GOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med 2006; 100:616–621.
- Nelson H, Weiss S, Bleecker E, Yancey S, Dorinsky P. The Salmeterol Multicenter Asthma Research Trial. Chest 2006; 129:15–26.
- Wechsler M, Lehman E, Lazarus S, et al. ß-Adrenergic receptor polymorphisms and response to salmeterol. Am J Respir Crit Care Med 2006; 173:519–526.
- Palmer CNA, Lipworth BJ, Lee S, Ismail T, MacGregor DF, Mukhopadhyay S. Arginine-16 beta-2 adrenoceptor genotype predisposes to exacerbations in young asthmatics taking regular salmeterol. Thorax 2006; 61:940–944.
- Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of beta 2 adrenoceptor polymorphism. Thorax 2000; 55:762–727.
- Bleecker E, Postma D, Lawrance R, Meyers D, Ambrose H, Goldman M. Effect of ADRB2 polymorphisms on response to long-acting beta2-agonist therapy: a pharmacogenetic analysis of two randomized studies. Lancet 2007; 370:2118–2125.
- Bleecker E, Yancey S, Baitinger L, et al. Salmeterol response is not affected by beta-2 adrenergic receptor genotype in subjects with persistent asthma. J Allergy Clin Immunol 2006; 118:809–816.
- Nelson H, Bleecker E, Corren J, et al. Characterization of asthma exacerbations by Arg16Gly genotype in subjects with asthma receiving salmeterol alone or with fluticasone propionate. J Allergy Clin Immunol 2008; 121:S131.
- O’Byrne P, Barnes P, Rodriguez-Roisin R, et al. Low dose Inhaled budesonide and formoterol in mild persistent asthma. The OPTIMA Randomized Trial. Am J Respir Crit Care Med 2001; 164:1392–1397.
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994; 344:219–224.
- Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med 1996; 153:1481–1488.
- Walters EH, Walters JAE, Gibson MDP. Long-acting beta2-agonists for stable chronic asthma. Cochrane Database Syst Rev 2003; (3):CD001385. doi:10.1002/14651858.CD001385.
- Masoli M, Weatherall M, Holt S, Beasley R. Moderate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroid in symptomatic asthma. Thorax 2005; 60:730–734.
- Sin DD, Man J, Sharpe H, Gan WQ, Man SFP. Pharmacological management to reduce exacerbations in adults with asthma. A systematic review and meta-analysis. JAMA 2004; 292:367–376.
- Mann RD, Kubota K, Pearce G, Wilton L. Salmeterol: a study by prescription event monitoring in a UK cohort of 15,407 patients. J Clin Epidemiol 1996; 49:247–250.
- Lanes S, Lanza L, Wentworth C. Risk of emergency care, hospitalization, and ICU stays for acute asthma among recipients of salmeterol. Am J Respir Crit Care Med 1998; 158:857–861.
- Meier CR, Jick H. Drug use and pulmonary death rates in increasingly symptomatic asthma patients in the UK. Thorax 1997; 52:612–617.
- Williams C, Crossland L, Finnerty J, et al. A case control study of salmeterol and near-fatal attacks of asthma. Thorax 1998; 53:7–13.
- Lanes S, Garcia Rodriguez LA, Herta C. Respiratory medications and risk of asthma death. Thorax 2002; 57:683–686.
- Anderson HR, Ayres JG, Sturdy PM, et al. Bronchodilator treatment and deaths from asthma: case control study. Br Med J 2005; 330:117–124.
- Martinez FD. Safety of long-acting beta agonists—an urgent need to clear the air. N Engl J Med 2005; 353:2637–2639.
- Nelson HS. Long-acting beta-agonists in adult asthma: evidence that these drugs are safe. Prim Care Respir J 2006; 15:271–277.
- Lang DM. The long-acting beta agonist controversy: a critical examination of the evidence. Cleve Clin J Med 2006; 73:973–992.
- Rambasek T, Lang DM, Kavuru M. Omalizumab: where does it fit in current asthma management? Cleve Clin J Med 2004; 71:251–261.
- McGlashan D, Bochner B, Adelman D, et al. Down regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol 1997; 158:1438–1445.
- Busse W, Corren J, Lanier B, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001; 108:184–190.
- Soler M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces asthma exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001; 18:254–261.
- Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005; 60:309–316.
- Van Ganse E, Antonicelli L, Zhang Q, et al. Asthma-related resource use and cost by GINA classification of severity in three European countries. Respir Med 2006; 100:140–147.
- Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J 2002; 19:61–67.
- Cisternas MG, Blanc PH, Yen IH, et al. A comprehensive study of the direct and indirect costs of adult asthma. J Allergy Clin Immunol 2003; 111:1212–1218.
- Sullivan S, Turk F. An evaluation of the cost effectiveness of omalizumab for the treatment of severe persistent asthma. Allergy 2008; 63:670–684.
- US Food and Drug Administration. Omalizumab (marketed as Xolair) information. www.fda.gov/cder/drug/infopage/omalizumab/default.htm. Accessed August 31, 2007.
- Williams SG, Schmidt DK, Redd SC, Storms W. Key clinical activities for quality asthma care. Recommendations of the National Asthma Education and Prevention Program. MMWR Recomm Rep 2003; 52 RR-6:1–8.
- Cox L, Li J, Nelson H, Lockey R, et al. Allergy Immunotherapy: a practice parameter second update. J Allergy Clin Immunol 2007; 120:S25–S85.
- Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol 2007; 119:780–789.
- Murray AB, Milner RA. The accuracy of features in the clinical history for predicting atopic sensitization to airborne allergens in children. J Allergy Clin Immunol 1995; 96:588–596.
- Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100 suppl 3:1S–148S.
- Walker S, Pajno GB, Lima MT, Wilson DR, Durham SR. Grass pollen immunotherapy for seasonal rhinitis and asthma: a randomized, controlled trial. J Allergy Clin Immunol 2001; 107:87–93.
- Varney VA, Edwards J, Tabbah K, Brewster H, Mavroleon G, Frew AJ. Clinical efficacy of specific immunotherapy to cat dander: a double-blind placebo-controlled trial. Clin Exp Allergy 1997; 27:860–867.
- Cantani A, Arcese G, Lucenti P, Gagliesi D, Bartolucci M. A three-year prospective study of specific immunotherapy to inhalant allergens: evidence of safety and efficacy in 300 children with allergic asthma. J Investig Allergol Clin Immunol 1997; 7:90–97.
- Hedlin G, Wille S, Browaldh L, et al. Immunotherapy in children with allergic asthma: effect on bronchial hyperreactivity and pharmacotherapy. J Allergy Clin Immunol 1999; 103:609–614.
- Arvidsson MB, Löwhagen O, Rak S. Allergen specific immunotherapy attenuates early and late phase reactions in lower airways of birch pollen asthmatic patients: a double blind placebo-controlled study. Allergy 2004; 59:74–80.
- Pichler CE, Helbling A, Pichler WJ. Three years of specific immunotherapy with house-dust-mite extracts in patients with rhinitis and asthma: significant improvement of allergen-specific parameters and of nonspecific bronchial hyperreactivity. Allergy 2001; 56:301–306.
- Mirone C, Albert F, Tosi A, et al. Efficacy and safety of subcutaneous immunotherapy with a biologically standardized extract of Ambrosia artemisiifolia pollen: a double-blind, placebo-controlled study. Clin Exp Allergy 2004; 34:1408–1414.
- Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev 2003; (4):CD001186.
- Jacobsen L. Preventive aspects of immunotherapy: prevention for children at risk of developing asthma. Ann Allergy Asthma Immunol 2001; 87:43–46.
- Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT study). J Allergy Clin Immunol 2002; 109:251–256.
- Niggemann B, Jacobsen L, Dreborg S, et al; PAT Investigator Group. Five year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy 2006: 61:855–859.
- Des Roches A, Paradis L, Menardo JL, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract VI: specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol 1997; 99:450–453.
- Pajno GB, Barberio G, DeLuca F, et al. Prevention of new sensitizations in asthmatic children monosensitized to the house dust mite by specific immunotherapy: a six year follow up study. Clin Exp Allergy 2001; 31:1392–1397.
- Lang DM. Do beta blockers really enhance the risk of anaphylaxis during immunotherapy? Curr Allergy Asthma Rep 2008; 8:37–44.
This review focuses on several elements in the National Asthma Education and Prevention Program’s new guidelines, the third Expert Panel Report (EPR3),1 that differ substantially from those in EPR2,2 issued in 1997 and updated in 2002.3 These differences in approach to the management of asthma described in EPR3 offer a clear potential for reducing the gap between optimal asthma care outcomes as described in guidelines and normative asthma care outcomes in the “real world.”
GREATER EMPHASIS ON CONTROL
The EPR2 guidelines2 recommended that asthma management be carried out in an algorithmic manner. Patients were classified into four severity categories: mild intermittent, mild persistent, moderate persistent, and severe persistent asthma, based on assessment of the level of symptoms (day/night), reliance on “reliever” medication, and lung function at the time of presentation. Pharmacologic management was then assigned according to each respective categorization in an evidence-based fashion.
In an ideal world, this would result in patients with asthma receiving appropriate pharmacotherapeutic agents associated with favorable asthma care outcomes, which were also advantageous from both cost- and risk-benefit standpoints. In the real world, however, this paradigm was flawed, as it relied on accurate categorization of patients in order for pharmacotherapy to be prescribed appropriately. Both providers and patients are prone to underestimate asthma severity,4,5 and for this reason many patients managed on the basis of this paradigm were undertreated.
A new paradigm, based on the assessment of asthma control, has been encouraged in the EPR3 guidelines.1
Severity and control are not synonymous
More than a decade ago, Cockroft and Swystun6 pointed out that asthma control (or lack thereof) is often used inappropriately to define asthma severity: ie, well-controlled asthma is seen as synonymous with mild asthma, and poorly controlled asthma with severe asthma.
Asthma severity can be defined as the intrinsic intensity of the disease process, while asthma control is the degree to which the manifestations of asthma are minimized. Asthma severity is clearly a determinant of asthma control, but its impact is affected by a variety of factors, including but not limited to:
- Whether appropriate medication is prescribed
- Patterns of therapeutic adherence
- The degree to which recommended measures for avoiding for clinically relevant aeroallergens are pursued.
Health care utilization, including hospitalizations and emergency department visits, correlates more closely with asthma control than with asthma severity.7–9 Indeed, a patient with severe persistent asthma who is treated appropriately with multiple “controller” medications and who takes his or her medications and avoids allergens as directed can achieve well-controlled or totally controlled asthma, and is not likely to require hospitalization or emergency department management, to miss school or work, or to experience nocturnal awakening or limitation in routine activities due to asthma. This patient has severe persistent asthma that is well controlled.
In contrast, a patient with mild or moderate persistent asthma who does not receive appropriate instructions for avoiding allergens or taking controller medication regularly or who is poorly adherent will likely have poor asthma control. This patient is more likely to require hospitalization or emergency department management, to miss school or work, and to experience nocturnal awakening or limitation in routine activities due to asthma. This patient has mild persistent asthma that is poorly controlled.
Assess asthma severity in the first visit, and control in subsequent visits
How to assess severity
How to measure control
For all patients with asthma, regardless of severity, the goal is the same: to achieve control by reducing both impairment and risk. Asthma is classified as well controlled, not well controlled, or poorly controlled (Table 2).1
Validated tests are available to assess control
Serial testing as a quality indicator
Serial ACT scores give an objective measure of the degree to which the goals of management1 are being achieved, and in so doing can encourage optimal outcomes.14
Another use of these tests is to document whether asthma control improves over time when patients receive care from a particular physician or group. This use may become increasingly important in view of efforts underway to implement a pay-for-performance model for asthma care, in which providers will be financially rewarded for improved patient care outcomes and adherence to standards of practice based on Health Plan Employer Data and Information Set measures.15
We have used the ACT in the Section of Allergy/Immunology at Cleveland Clinic for 3 years on a routine basis. All patients with asthma being seen either for the first time or as follow-up complete the ACT, which has been entered in a flow sheet in our electronic medical record, at the same time they undergo spirometry. We have shown that care in the Section of Allergy/Immunology is associated with improvement in asthma control over time, in patients who have completed serial ACT measurements at initial visits and at follow-up visits (Figure 2).
Objective measurement of lung function is also important
Serial monitoring of lung function at every patient visit with spirometry is also important, as some patients may be “poor perceivers,”16 ie, they may have little or no subjective awareness of moderate or even severe ventilatory impairment. A number of studies17,18 support the contention that symptoms and lung function are separate and independent dimensions of asthma control, and that both of them need to be assessed.
Responding to changes in control
THE STEP 3 CONTROVERSY
Salmeterol Multicenter Asthma Research Trial
In the Salmeterol Multicenter Asthma Research Trial (SMART), patients randomized to the long-acting beta agonist (LABA) salmeterol (Serevent)—particularly African Americans—had a statistically significant increase in the risk of untoward asthma care outcomes.20
SMART was launched in 1996. Patients were randomized in a double-blind fashion to receive either salmeterol 42 μg twice a day or placebo in addition to their usual asthma therapy for 28 weeks. The rate of the primary outcome (respiratory-related deaths or life-threatening experiences) was not significantly different with salmeterol than with placebo (relative risk [RR] = 1.40, 95% confidence interval [CI] 0.91–2.14). However, in 2003, the study was halted prematurely because of difficulty enrolling the targeted number of 60,000 patients, and an interim analysis that revealed significantly higher rates of secondary outcomes in subjects randomized to salmeterol. Compared with the placebo group, the salmeterol group had significantly higher rates of respiratory-related deaths (RR 2.16, 95% CI 1.06–4.41), asthma-related deaths (RR = 4.37, 95% CI = 1.25–15.34), and combined asthma-related deaths or life-threatening experiences (RR = 1.71, 95% CI 1.01–2.89). There were 13 asthma-related deaths and 37 combined asthma-related deaths or life-threatening experiences in the salmeterol group, compared with 3 and 22, respectively, in the placebo group. Of the 16 asthma deaths in the study, 13 (81%) occurred in the initial phase of SMART, when patients were recruited via print, radio, and television advertising; afterward, patients were recruited directly by investigators.
Statistically significant differences in outcomes occurred primarily in African Americans. African Americans who received salmeterol had higher rates of respiratory death or life-threatening experiences (RR = 4.10, 95% CI 1.54–10.90), the primary end point for the study, as well as higher rates of combined asthma-related deaths or life-threatening experiences (RR = 10.46, 95% CI 1.34–81.58), a secondary end point. No statistically significant differences were observed in white patients randomized to salmeterol with respect to the primary end point (RR = 1.05, 95% = 0.62–1.76); the secondary end point of combined asthma-related deaths or life-threatening experiences (RR = 1.08, 95% CI 0.55–2.14); or other end points.
Medication exposures were not tracked during the study, and allocation to inhaled corticosteroids combined with salmeterol was not randomized, so the effect of concomitant inhaled corticosteroid use cannot be determined from these data.
As a result of SMART, medications that contain either of the two LABAs, salmeterol or formoterol (Foradil), carry a black-box warning.
LABAs: Risks and benefits
Two studies21,22 have suggested that asthmatic patients who are homozygous for Arg/Arg at codon 16 of the beta-2 adrenergic receptor are predisposed to untoward asthma outcomes with regular exposure to LABAs. However, other data23–25 do not support the contention that B16 Arg/Arg patients experience adverse asthma outcomes with LABA exposure. In two recently published studies, no difference in rates of exacerbations, severe exacerbations, lung function, frequency of reliance on SABA, or nocturnal awakenings was observed in patients receiving formoterol combined with budesonide24 or salmeterol combined with fluticasone25 according to genotype. A prospective study26 also found no statistically significant difference in exacerbation rates according to beta adrenergic receptor genotype in individuals randomized to LABA monotherapy, or LABA combined with inhaled corticosteroids.
The updated EPR2 asthma guidelines,3 published in November 2002, stipulated that LABAs were the preferred controller agent to “add on” to low-dose inhaled corticosteroids for patients with moderate persistent asthma, and that the combination of low-dose inhaled corticosteroids and LABA was associated with superior outcomes: reduction of symptoms, including nocturnal awakening, increase in lung function, improvement in health-related quality of life, decreased use of “rescue” medication, and reduced rate of exacerbations and severe exacerbations, compared with higher-dose inhaled corticosteroid monotherapy. This management recommendation was categorized as level A, on the basis of data from multiple randomized, controlled, double-blinded trials.27–29 Additional evidence14,30 and data from two meta-analyses31,32 have provided further support for this recommendation, while no evidence linking LABA exposure to risk for fatal or near-fatal asthma has been found in cohort or case-control studies.33–38
Based on safety concerns, the EPR3 guidelines1 recommend that medium-dose inhaled corticosteroids be regarded as equivalent to adding LABAs to low-dose inhaled corticosteroids, and state: “the established, beneficial effects of LABA for the great majority of patients whose asthma is not well controlled with [inhaled corticosteroids] alone should be weighed against the increased risk for severe exacerbations, although uncommon, associated with daily use of LABA.”1
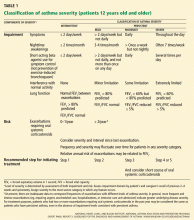
There is currently an honest difference of opinion39,40 among asthma specialists as to how this management recommendation for moderate persistent asthma—now depicted at “step 3” in the EPR3 guidelines (Table 4)—should be implemented. The LABA controversy was reviewed previously in the Cleveland Clinic Journal of Medicine.41
THE ROLE OF OMALIZUMAB: WEIGHING COST VS BENEFIT
The 2002 update to the EPR2 guidelines3 was issued before omalizumab (Xolair) was approved in June 2003.
Patients with severe persistent asthma are categorized in steps 5 or 6 in the EPR3 guidelines (Table 5).1 Preferred management for these patients includes inhaled corticosteroids in high doses combined with long-acting beta agonists and, for step 6 patients, oral corticosteroids.
Omalizumab was approved for management of patients with moderate or severe persistent asthma who are not achieving the goals of asthma management on inhaled corticosteroids, who exhibit a wheal-flare reaction to a perennial allergen, and whose immunoglobulin E (IgE) level is in the range of 30 to 700 IU/mL.42 Omalizumab dosing is based on the serum IgE level and on body weight.
Omalizumab, an anti-IgE monoclonal antibody
Omalizumab is a recombinant, humanized, monoclonal anti-IgE antibody that binds to IgE at the same Fc site as the high-affinity IgE receptor. Its primary mechanism of action is the binding of free IgE in the circulation, forming biologically inert, small complexes that do not activate complement and are cleared by the reticuloendothelial system.42 Its secondary mechanism of action entails a reduction in the number of high-affinity receptors on basophils, from approximately 220,000 to 8,300 receptors per cell. The latter effect was associated with a 90% reduction in histamine release from basophils in response to ex vivo challenge with dust mite allergen.43
Benefit in randomized trials
Omalizumab has been associated with statistically and clinically significant benefit in randomized, double-blind, placebo-controlled trials.44,45
Humbert et al46 randomized 419 patients whose asthma was not adequately controlled on high-dose inhaled corticosteroids and long-acting beta agonists, who were 12 to 75 years old, with reduced lung function and a history of recent asthma exacerbation, to treatment with omalizumab or placebo. Omalizumab was associated with a statistically significant reduction in the rate of asthma exacerbations and severe asthma exacerbations, as well as statistically significant improvements in asthma-related quality of life, morning peak expiratory flow rate, and asthma symptom scores.
These data support the recommendation in EPR3 to consider a trial of omalizumab in properly selected patients with severe, persistent allergic asthma.
Omalizumab is cost-beneficial in properly selected patients
The current wholesale acquisition cost of omalizumab is $532 for one 150-mg vial (David Zito, personal communication). The cost of treatment varies based on body weight and IgE level but may range from a wholesale cost of $6,388 to $38,326 per year.
However, as asthma severity increases, both direct and indirect medical expenditures increase substantially.47,48 Annual costs are approximately four times higher for severe asthma compared with mild asthma49; not only are treatment and exacerbation costs higher, but indirect costs are also disproportionately greater. Annual costs for severe asthma are significantly greater if the disease is inadequately controlled.50 For these reasons, an intervention that leads to improved outcomes for severe, poorly controlled asthma carries the potential for the greatest cost-utility for society, as it can lower direct costs by reducing the frequency and severity of exacerbations, in addition to reducing indirect medical expenditures on the basis of increased productivity and fewer days of missed work or school. The cost of omalizumab in quality-adjusted life years compares favorably with that of biologicals used in managing rheumatoid arthritis, Crohn disease, and multiple sclerosis.50
Adverse effects of omalizumab
In pivotal trials,43,44 omalizumab was associated with a substantial rate of local reactions. The rate of anaphylaxis was slightly less than 1 in 1,000, and this has been confirmed by surveillance data recorded since approval of the drug in 2003. Based on the observed risk of anaphylaxis, in July 2007, the US Food and Drug Administration added a black-box warning to the omalizumab label and stipulated that a medication guide should be provided for patients.51 The warning indicates that health care providers administering omalizumab should be prepared to manage anaphylaxis and that patients should be closely observed for an appropriate period after omalizumab administration.
The package insert also describes a numerical, but not statistically significant, increase in the rate of malignancy in patients receiving omalizumab.42 Malignancy developed in 0.5% of patients receiving omalizumab, compared with 0.2% of patients who received placebo. Because these malignancies were diagnosed over a shorter period than the time required for oncogenesis (ie, 6 months in 60% of cases), and because a heterogeneous variety of tumors was observed, there is reason to doubt these tumors were causally associated with omalizumab.
Postmarketing surveillance studies are in progress that will provide more definitive data on the potential relationship between malignancy and omalizumab exposure.
Omalizumab: Guideline recommendations
The EPR3 guidelines1 state that omalizumab is the only adjunctive therapy to demonstrate efficacy when added to high-dose inhaled corticosteroids plus long-acting beta agonists in patients with severe, persistent, allergic asthma and that evidence does not support use of the following agents, which in some cases are approved for managing other conditions and have been advocated for management of severe, refractory asthma: methotrexate, soluble interleukin (IL)-4 receptor, anti-IL-5, anti-IL-12, cyclosporine A, intravenous immune globulin, gold, troleandomycin, and colchicine. The data supporting use of macrolides were characterized as “encouraging but insufficient to support a recommendation.”
The strength of evidence for the use of omalizumab for patients in steps 5 and 6 who fulfill the criteria for its use (see above) was classified in the EPR3 guidelines1 as category B. The guidelines also say that omalizumab may be considered for adjunctive therapy in properly selected patients in step 4, as a means to avoid higher doses of inhaled corticosteroids, but that additional studies are needed to establish its utility for such patients. This recommendation was classified as category D because of the lack of published comparator trials.
ALLERGEN IMMUNOTHERAPY FOR PATIENTS WITH ASTHMA
Many patients with asthma have clinically relevant, IgE-mediated (allergic) potential to inhaled allergens.1 For patients with persistent asthma (steps 2–6 in Table 5), allergic reactions can contribute to airway inflammation, provoke symptoms, and lead to more use of medications. For this reason, identification and management of clinically relevant allergy merits consideration.52
The EPR3 guidelines1 recommend considering allergen immunotherapy for patients with mild or moderate persistent asthma (steps 2–4) who have a clinically relevant component of allergy to inhaled substances.
Changing the immune response
Allergen immunotherapy entails the incremental administration of inhalant allergens by subcutaneous injection for the purpose of inducing immune system changes in the host response. The goal of immunotherapy is to protect against allergic reactions that can be expected to occur with ongoing exposure to clinically relevant allergens.53
The immunologic changes that develop with allergen immunotherapy are complex.53,54 Successful immunotherapy results in generation of a population of CD4+/CD25+ T lymphocytes producing IL-10, transforming growth factor beta, or both. Allergen immunotherapy has been shown to block the immediate- and late-phase allergic response; to decrease recruitment of mast cells, basophils, and eosinophils on provocation or natural exposure to allergens in the skin, nose, eye, and bronchial mucosa; to blunt the seasonal rise in specific IgE; and to suppress late-phase inflammatory responses in the skin and respiratory tract. However, the efficacy of immunotherapy in relation to these immunologic changes is not completely understood.54
Many patients need skin testing
Allergen immunotherapy may be considered for patients with asthma for whom a clear relationship exists between symptoms and exposure to an allergen to which the patient is sensitive.53 Because it is often not possible to determine whether a patient is sensitive to a perennial indoor allergen (eg, dust mite) on the basis of the medical history alone,55 many patients with asthma benefit from immediate hypersensitivity skin testing to objectively assess or rule out allergy to common inhalants. In certain situations, in vitro testing may be performed, but skin testing has a higher negative predictive value and is recommended as a better screening test.56
Benefits of allergen immunotherapy
Numerous randomized, double-blind, placebo-controlled trials have shown that allergen immunotherapy is associated with benefit for reducing symptoms and medication reliance.57–63
A meta-analysis of 75 randomized, placebo-controlled studies confirmed the effectiveness of immunotherapy in asthma, with a significant reduction in asthma symptoms and medication use and with improvement in bronchial hyperreactivity.64 This meta-analysis included 36 trials of dust mite allergen, 20 of pollen, and 10 of animal dander. Immunotherapy is efficacious for pollen, mold, dust mite, cockroach, and animal allergens; however, its effectiveness is more established for dust mite, animal dander, and pollen allergens, as fewer studies have been published demonstrating efficacy using mold and cockroach allergens.53
In addition, several studies have found that children with allergic rhinitis who receive allergen immunotherapy are significantly less likely to develop asthma.65–67 Immunotherapy has also been associated with a statistically significant reduction in future sensitization to other aeroallergens.68,69
Risk of systemic reaction from allergen immunotherapy
The decision to begin allergen immunotherapy should be individualized on the basis of symptom severity, relative benefit compared with drug therapy, and whether comorbid conditions such as cardiovascular disease or beta-blocker exposure are present. These comorbid conditions are associated with heightened risk of (more serious) anaphylaxis—the major hazard of allergen immunotherapy.70 Systemic reactions during allergen immunotherapy occur at a rate of approximately 3 to 5 per 1,000 injections; for this reason, allergen immunotherapy should only be administered in a medical facility where personnel, supplies, and equipment are available to treat anaphylaxis.5
This review focuses on several elements in the National Asthma Education and Prevention Program’s new guidelines, the third Expert Panel Report (EPR3),1 that differ substantially from those in EPR2,2 issued in 1997 and updated in 2002.3 These differences in approach to the management of asthma described in EPR3 offer a clear potential for reducing the gap between optimal asthma care outcomes as described in guidelines and normative asthma care outcomes in the “real world.”
GREATER EMPHASIS ON CONTROL
The EPR2 guidelines2 recommended that asthma management be carried out in an algorithmic manner. Patients were classified into four severity categories: mild intermittent, mild persistent, moderate persistent, and severe persistent asthma, based on assessment of the level of symptoms (day/night), reliance on “reliever” medication, and lung function at the time of presentation. Pharmacologic management was then assigned according to each respective categorization in an evidence-based fashion.
In an ideal world, this would result in patients with asthma receiving appropriate pharmacotherapeutic agents associated with favorable asthma care outcomes, which were also advantageous from both cost- and risk-benefit standpoints. In the real world, however, this paradigm was flawed, as it relied on accurate categorization of patients in order for pharmacotherapy to be prescribed appropriately. Both providers and patients are prone to underestimate asthma severity,4,5 and for this reason many patients managed on the basis of this paradigm were undertreated.
A new paradigm, based on the assessment of asthma control, has been encouraged in the EPR3 guidelines.1
Severity and control are not synonymous
More than a decade ago, Cockroft and Swystun6 pointed out that asthma control (or lack thereof) is often used inappropriately to define asthma severity: ie, well-controlled asthma is seen as synonymous with mild asthma, and poorly controlled asthma with severe asthma.
Asthma severity can be defined as the intrinsic intensity of the disease process, while asthma control is the degree to which the manifestations of asthma are minimized. Asthma severity is clearly a determinant of asthma control, but its impact is affected by a variety of factors, including but not limited to:
- Whether appropriate medication is prescribed
- Patterns of therapeutic adherence
- The degree to which recommended measures for avoiding for clinically relevant aeroallergens are pursued.
Health care utilization, including hospitalizations and emergency department visits, correlates more closely with asthma control than with asthma severity.7–9 Indeed, a patient with severe persistent asthma who is treated appropriately with multiple “controller” medications and who takes his or her medications and avoids allergens as directed can achieve well-controlled or totally controlled asthma, and is not likely to require hospitalization or emergency department management, to miss school or work, or to experience nocturnal awakening or limitation in routine activities due to asthma. This patient has severe persistent asthma that is well controlled.
In contrast, a patient with mild or moderate persistent asthma who does not receive appropriate instructions for avoiding allergens or taking controller medication regularly or who is poorly adherent will likely have poor asthma control. This patient is more likely to require hospitalization or emergency department management, to miss school or work, and to experience nocturnal awakening or limitation in routine activities due to asthma. This patient has mild persistent asthma that is poorly controlled.
Assess asthma severity in the first visit, and control in subsequent visits
How to assess severity
How to measure control
For all patients with asthma, regardless of severity, the goal is the same: to achieve control by reducing both impairment and risk. Asthma is classified as well controlled, not well controlled, or poorly controlled (Table 2).1
Validated tests are available to assess control
Serial testing as a quality indicator
Serial ACT scores give an objective measure of the degree to which the goals of management1 are being achieved, and in so doing can encourage optimal outcomes.14
Another use of these tests is to document whether asthma control improves over time when patients receive care from a particular physician or group. This use may become increasingly important in view of efforts underway to implement a pay-for-performance model for asthma care, in which providers will be financially rewarded for improved patient care outcomes and adherence to standards of practice based on Health Plan Employer Data and Information Set measures.15
We have used the ACT in the Section of Allergy/Immunology at Cleveland Clinic for 3 years on a routine basis. All patients with asthma being seen either for the first time or as follow-up complete the ACT, which has been entered in a flow sheet in our electronic medical record, at the same time they undergo spirometry. We have shown that care in the Section of Allergy/Immunology is associated with improvement in asthma control over time, in patients who have completed serial ACT measurements at initial visits and at follow-up visits (Figure 2).
Objective measurement of lung function is also important
Serial monitoring of lung function at every patient visit with spirometry is also important, as some patients may be “poor perceivers,”16 ie, they may have little or no subjective awareness of moderate or even severe ventilatory impairment. A number of studies17,18 support the contention that symptoms and lung function are separate and independent dimensions of asthma control, and that both of them need to be assessed.
Responding to changes in control
THE STEP 3 CONTROVERSY
Salmeterol Multicenter Asthma Research Trial
In the Salmeterol Multicenter Asthma Research Trial (SMART), patients randomized to the long-acting beta agonist (LABA) salmeterol (Serevent)—particularly African Americans—had a statistically significant increase in the risk of untoward asthma care outcomes.20
SMART was launched in 1996. Patients were randomized in a double-blind fashion to receive either salmeterol 42 μg twice a day or placebo in addition to their usual asthma therapy for 28 weeks. The rate of the primary outcome (respiratory-related deaths or life-threatening experiences) was not significantly different with salmeterol than with placebo (relative risk [RR] = 1.40, 95% confidence interval [CI] 0.91–2.14). However, in 2003, the study was halted prematurely because of difficulty enrolling the targeted number of 60,000 patients, and an interim analysis that revealed significantly higher rates of secondary outcomes in subjects randomized to salmeterol. Compared with the placebo group, the salmeterol group had significantly higher rates of respiratory-related deaths (RR 2.16, 95% CI 1.06–4.41), asthma-related deaths (RR = 4.37, 95% CI = 1.25–15.34), and combined asthma-related deaths or life-threatening experiences (RR = 1.71, 95% CI 1.01–2.89). There were 13 asthma-related deaths and 37 combined asthma-related deaths or life-threatening experiences in the salmeterol group, compared with 3 and 22, respectively, in the placebo group. Of the 16 asthma deaths in the study, 13 (81%) occurred in the initial phase of SMART, when patients were recruited via print, radio, and television advertising; afterward, patients were recruited directly by investigators.
Statistically significant differences in outcomes occurred primarily in African Americans. African Americans who received salmeterol had higher rates of respiratory death or life-threatening experiences (RR = 4.10, 95% CI 1.54–10.90), the primary end point for the study, as well as higher rates of combined asthma-related deaths or life-threatening experiences (RR = 10.46, 95% CI 1.34–81.58), a secondary end point. No statistically significant differences were observed in white patients randomized to salmeterol with respect to the primary end point (RR = 1.05, 95% = 0.62–1.76); the secondary end point of combined asthma-related deaths or life-threatening experiences (RR = 1.08, 95% CI 0.55–2.14); or other end points.
Medication exposures were not tracked during the study, and allocation to inhaled corticosteroids combined with salmeterol was not randomized, so the effect of concomitant inhaled corticosteroid use cannot be determined from these data.
As a result of SMART, medications that contain either of the two LABAs, salmeterol or formoterol (Foradil), carry a black-box warning.
LABAs: Risks and benefits
Two studies21,22 have suggested that asthmatic patients who are homozygous for Arg/Arg at codon 16 of the beta-2 adrenergic receptor are predisposed to untoward asthma outcomes with regular exposure to LABAs. However, other data23–25 do not support the contention that B16 Arg/Arg patients experience adverse asthma outcomes with LABA exposure. In two recently published studies, no difference in rates of exacerbations, severe exacerbations, lung function, frequency of reliance on SABA, or nocturnal awakenings was observed in patients receiving formoterol combined with budesonide24 or salmeterol combined with fluticasone25 according to genotype. A prospective study26 also found no statistically significant difference in exacerbation rates according to beta adrenergic receptor genotype in individuals randomized to LABA monotherapy, or LABA combined with inhaled corticosteroids.
The updated EPR2 asthma guidelines,3 published in November 2002, stipulated that LABAs were the preferred controller agent to “add on” to low-dose inhaled corticosteroids for patients with moderate persistent asthma, and that the combination of low-dose inhaled corticosteroids and LABA was associated with superior outcomes: reduction of symptoms, including nocturnal awakening, increase in lung function, improvement in health-related quality of life, decreased use of “rescue” medication, and reduced rate of exacerbations and severe exacerbations, compared with higher-dose inhaled corticosteroid monotherapy. This management recommendation was categorized as level A, on the basis of data from multiple randomized, controlled, double-blinded trials.27–29 Additional evidence14,30 and data from two meta-analyses31,32 have provided further support for this recommendation, while no evidence linking LABA exposure to risk for fatal or near-fatal asthma has been found in cohort or case-control studies.33–38
Based on safety concerns, the EPR3 guidelines1 recommend that medium-dose inhaled corticosteroids be regarded as equivalent to adding LABAs to low-dose inhaled corticosteroids, and state: “the established, beneficial effects of LABA for the great majority of patients whose asthma is not well controlled with [inhaled corticosteroids] alone should be weighed against the increased risk for severe exacerbations, although uncommon, associated with daily use of LABA.”1
There is currently an honest difference of opinion39,40 among asthma specialists as to how this management recommendation for moderate persistent asthma—now depicted at “step 3” in the EPR3 guidelines (Table 4)—should be implemented. The LABA controversy was reviewed previously in the Cleveland Clinic Journal of Medicine.41
THE ROLE OF OMALIZUMAB: WEIGHING COST VS BENEFIT
The 2002 update to the EPR2 guidelines3 was issued before omalizumab (Xolair) was approved in June 2003.
Patients with severe persistent asthma are categorized in steps 5 or 6 in the EPR3 guidelines (Table 5).1 Preferred management for these patients includes inhaled corticosteroids in high doses combined with long-acting beta agonists and, for step 6 patients, oral corticosteroids.
Omalizumab was approved for management of patients with moderate or severe persistent asthma who are not achieving the goals of asthma management on inhaled corticosteroids, who exhibit a wheal-flare reaction to a perennial allergen, and whose immunoglobulin E (IgE) level is in the range of 30 to 700 IU/mL.42 Omalizumab dosing is based on the serum IgE level and on body weight.
Omalizumab, an anti-IgE monoclonal antibody
Omalizumab is a recombinant, humanized, monoclonal anti-IgE antibody that binds to IgE at the same Fc site as the high-affinity IgE receptor. Its primary mechanism of action is the binding of free IgE in the circulation, forming biologically inert, small complexes that do not activate complement and are cleared by the reticuloendothelial system.42 Its secondary mechanism of action entails a reduction in the number of high-affinity receptors on basophils, from approximately 220,000 to 8,300 receptors per cell. The latter effect was associated with a 90% reduction in histamine release from basophils in response to ex vivo challenge with dust mite allergen.43
Benefit in randomized trials
Omalizumab has been associated with statistically and clinically significant benefit in randomized, double-blind, placebo-controlled trials.44,45
Humbert et al46 randomized 419 patients whose asthma was not adequately controlled on high-dose inhaled corticosteroids and long-acting beta agonists, who were 12 to 75 years old, with reduced lung function and a history of recent asthma exacerbation, to treatment with omalizumab or placebo. Omalizumab was associated with a statistically significant reduction in the rate of asthma exacerbations and severe asthma exacerbations, as well as statistically significant improvements in asthma-related quality of life, morning peak expiratory flow rate, and asthma symptom scores.
These data support the recommendation in EPR3 to consider a trial of omalizumab in properly selected patients with severe, persistent allergic asthma.
Omalizumab is cost-beneficial in properly selected patients
The current wholesale acquisition cost of omalizumab is $532 for one 150-mg vial (David Zito, personal communication). The cost of treatment varies based on body weight and IgE level but may range from a wholesale cost of $6,388 to $38,326 per year.
However, as asthma severity increases, both direct and indirect medical expenditures increase substantially.47,48 Annual costs are approximately four times higher for severe asthma compared with mild asthma49; not only are treatment and exacerbation costs higher, but indirect costs are also disproportionately greater. Annual costs for severe asthma are significantly greater if the disease is inadequately controlled.50 For these reasons, an intervention that leads to improved outcomes for severe, poorly controlled asthma carries the potential for the greatest cost-utility for society, as it can lower direct costs by reducing the frequency and severity of exacerbations, in addition to reducing indirect medical expenditures on the basis of increased productivity and fewer days of missed work or school. The cost of omalizumab in quality-adjusted life years compares favorably with that of biologicals used in managing rheumatoid arthritis, Crohn disease, and multiple sclerosis.50
Adverse effects of omalizumab
In pivotal trials,43,44 omalizumab was associated with a substantial rate of local reactions. The rate of anaphylaxis was slightly less than 1 in 1,000, and this has been confirmed by surveillance data recorded since approval of the drug in 2003. Based on the observed risk of anaphylaxis, in July 2007, the US Food and Drug Administration added a black-box warning to the omalizumab label and stipulated that a medication guide should be provided for patients.51 The warning indicates that health care providers administering omalizumab should be prepared to manage anaphylaxis and that patients should be closely observed for an appropriate period after omalizumab administration.
The package insert also describes a numerical, but not statistically significant, increase in the rate of malignancy in patients receiving omalizumab.42 Malignancy developed in 0.5% of patients receiving omalizumab, compared with 0.2% of patients who received placebo. Because these malignancies were diagnosed over a shorter period than the time required for oncogenesis (ie, 6 months in 60% of cases), and because a heterogeneous variety of tumors was observed, there is reason to doubt these tumors were causally associated with omalizumab.
Postmarketing surveillance studies are in progress that will provide more definitive data on the potential relationship between malignancy and omalizumab exposure.
Omalizumab: Guideline recommendations
The EPR3 guidelines1 state that omalizumab is the only adjunctive therapy to demonstrate efficacy when added to high-dose inhaled corticosteroids plus long-acting beta agonists in patients with severe, persistent, allergic asthma and that evidence does not support use of the following agents, which in some cases are approved for managing other conditions and have been advocated for management of severe, refractory asthma: methotrexate, soluble interleukin (IL)-4 receptor, anti-IL-5, anti-IL-12, cyclosporine A, intravenous immune globulin, gold, troleandomycin, and colchicine. The data supporting use of macrolides were characterized as “encouraging but insufficient to support a recommendation.”
The strength of evidence for the use of omalizumab for patients in steps 5 and 6 who fulfill the criteria for its use (see above) was classified in the EPR3 guidelines1 as category B. The guidelines also say that omalizumab may be considered for adjunctive therapy in properly selected patients in step 4, as a means to avoid higher doses of inhaled corticosteroids, but that additional studies are needed to establish its utility for such patients. This recommendation was classified as category D because of the lack of published comparator trials.
ALLERGEN IMMUNOTHERAPY FOR PATIENTS WITH ASTHMA
Many patients with asthma have clinically relevant, IgE-mediated (allergic) potential to inhaled allergens.1 For patients with persistent asthma (steps 2–6 in Table 5), allergic reactions can contribute to airway inflammation, provoke symptoms, and lead to more use of medications. For this reason, identification and management of clinically relevant allergy merits consideration.52
The EPR3 guidelines1 recommend considering allergen immunotherapy for patients with mild or moderate persistent asthma (steps 2–4) who have a clinically relevant component of allergy to inhaled substances.
Changing the immune response
Allergen immunotherapy entails the incremental administration of inhalant allergens by subcutaneous injection for the purpose of inducing immune system changes in the host response. The goal of immunotherapy is to protect against allergic reactions that can be expected to occur with ongoing exposure to clinically relevant allergens.53
The immunologic changes that develop with allergen immunotherapy are complex.53,54 Successful immunotherapy results in generation of a population of CD4+/CD25+ T lymphocytes producing IL-10, transforming growth factor beta, or both. Allergen immunotherapy has been shown to block the immediate- and late-phase allergic response; to decrease recruitment of mast cells, basophils, and eosinophils on provocation or natural exposure to allergens in the skin, nose, eye, and bronchial mucosa; to blunt the seasonal rise in specific IgE; and to suppress late-phase inflammatory responses in the skin and respiratory tract. However, the efficacy of immunotherapy in relation to these immunologic changes is not completely understood.54
Many patients need skin testing
Allergen immunotherapy may be considered for patients with asthma for whom a clear relationship exists between symptoms and exposure to an allergen to which the patient is sensitive.53 Because it is often not possible to determine whether a patient is sensitive to a perennial indoor allergen (eg, dust mite) on the basis of the medical history alone,55 many patients with asthma benefit from immediate hypersensitivity skin testing to objectively assess or rule out allergy to common inhalants. In certain situations, in vitro testing may be performed, but skin testing has a higher negative predictive value and is recommended as a better screening test.56
Benefits of allergen immunotherapy
Numerous randomized, double-blind, placebo-controlled trials have shown that allergen immunotherapy is associated with benefit for reducing symptoms and medication reliance.57–63
A meta-analysis of 75 randomized, placebo-controlled studies confirmed the effectiveness of immunotherapy in asthma, with a significant reduction in asthma symptoms and medication use and with improvement in bronchial hyperreactivity.64 This meta-analysis included 36 trials of dust mite allergen, 20 of pollen, and 10 of animal dander. Immunotherapy is efficacious for pollen, mold, dust mite, cockroach, and animal allergens; however, its effectiveness is more established for dust mite, animal dander, and pollen allergens, as fewer studies have been published demonstrating efficacy using mold and cockroach allergens.53
In addition, several studies have found that children with allergic rhinitis who receive allergen immunotherapy are significantly less likely to develop asthma.65–67 Immunotherapy has also been associated with a statistically significant reduction in future sensitization to other aeroallergens.68,69
Risk of systemic reaction from allergen immunotherapy
The decision to begin allergen immunotherapy should be individualized on the basis of symptom severity, relative benefit compared with drug therapy, and whether comorbid conditions such as cardiovascular disease or beta-blocker exposure are present. These comorbid conditions are associated with heightened risk of (more serious) anaphylaxis—the major hazard of allergen immunotherapy.70 Systemic reactions during allergen immunotherapy occur at a rate of approximately 3 to 5 per 1,000 injections; for this reason, allergen immunotherapy should only be administered in a medical facility where personnel, supplies, and equipment are available to treat anaphylaxis.5
- National Heart, Lung, and Blood institute, National Asthma education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. www.nhlbi.nih.gov/guidelines/asthma. Accessed 8/7/08.
- Expert Panel Report 2: Guidelines for the diagnosis and management of asthma. U.S. Department of Health and Human Services. Publication No. 97-4051; 1997.
- Expert Panel Report: Guidelines for the diagnosis and management of asthma. Update on Selected Topics—2002. J Allergy Clin Immunol 2002; 110:S141–S207.
- FitzGerald JM, Boulet LP, McIvor RA, Zimmerman S, Chapman KR. Asthma control in Canada remains suboptimal: the Reality of Asthma Control (TRAC) study. Can Respir J 2006; 13:253–259.
- Braganza S, Sharif I, Ozuah P. Documenting asthma severity: do we get it right? J Asthma 2003; 40:661–665.
- Cockcroft DW, Swystun VA. Asthma control versus asthma severity. J Allergy Clin Immunol 1996; 98:1016–1018.
- Peters SP, Jones CA, Haselkorn T, Mink DR, Valacer DJ, Weiss ST. Real-world Evaluation of Asthma Control and Treatment (REACT): findings from a national Web-based survey. J Allergy Clin Immunol. 2007; 119:1454–1461.
- Osborne ML, Vollmer WM, Pedula KL, Wilkins J, Buist AS, O’Hollaren M. Lack of correlation of symptoms with specialist-assessed long-term asthma severity. Chest 1999; 115:85–91.
- Li JT, Oppenheimer J, Bernstein IL, et al. Attaining optimal asthma control: a practice parameter. J Allergy Clin Immunol 2005; 116:S3–S11.
- Nathan RA, Sorkness C, Kosinski M, et al. Development of the Asthma Control Test: a survey for assessing asthma control. J Allergy Clin Immunol 2004; 113:59–65.
- Schatz M, Zeiger RS, Drane A, et al. Reliability and predictive validity of the Asthma Control Test administered by telephone calls using speech recognition technology. J Allergy Clin Immunol 2007; 119:336–343.
- Peters D, Chen C, Markson LE, Allen-Ramey FC, Vollmer WM. Using an asthma control questionnaire and administrative data to predict healthcare utilization. Chest 2006; 129:918–924.
- Schatz M, Sorkness C, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006; 117:549–556.
- Bateman E, Boushey H, Bousquet J, et al. Can guideline-defined asthma control be achieved? Am J Respir Crit Care Med 2004; 170:836–844.
- Davies TJ, Bunn WB, Fromer L, Gelfand EW, Colice GL. A focus on the asthma HEDIS measure and its implications for clinical practice. Manag Care Interface 2006; 19:29–36.
- Rubinfeld AR, Pain MC. Perception of asthma. Lancet 1976; 1:882–884.
- Teeter J, Bleecker E. Relationship between airway obstruction and respiratory symptoms in adult asthmatics. Chest 1998; 113:272–277.
- Shingo S, Zhang J, Reiss T. Correlation of airway obstruction and patient reported endpoints in clinical studies. Eur Resp J 2001; 17:220–224.
- Juniper EF, Bousquet J, Abetz L, Bateman ED; GOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med 2006; 100:616–621.
- Nelson H, Weiss S, Bleecker E, Yancey S, Dorinsky P. The Salmeterol Multicenter Asthma Research Trial. Chest 2006; 129:15–26.
- Wechsler M, Lehman E, Lazarus S, et al. ß-Adrenergic receptor polymorphisms and response to salmeterol. Am J Respir Crit Care Med 2006; 173:519–526.
- Palmer CNA, Lipworth BJ, Lee S, Ismail T, MacGregor DF, Mukhopadhyay S. Arginine-16 beta-2 adrenoceptor genotype predisposes to exacerbations in young asthmatics taking regular salmeterol. Thorax 2006; 61:940–944.
- Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of beta 2 adrenoceptor polymorphism. Thorax 2000; 55:762–727.
- Bleecker E, Postma D, Lawrance R, Meyers D, Ambrose H, Goldman M. Effect of ADRB2 polymorphisms on response to long-acting beta2-agonist therapy: a pharmacogenetic analysis of two randomized studies. Lancet 2007; 370:2118–2125.
- Bleecker E, Yancey S, Baitinger L, et al. Salmeterol response is not affected by beta-2 adrenergic receptor genotype in subjects with persistent asthma. J Allergy Clin Immunol 2006; 118:809–816.
- Nelson H, Bleecker E, Corren J, et al. Characterization of asthma exacerbations by Arg16Gly genotype in subjects with asthma receiving salmeterol alone or with fluticasone propionate. J Allergy Clin Immunol 2008; 121:S131.
- O’Byrne P, Barnes P, Rodriguez-Roisin R, et al. Low dose Inhaled budesonide and formoterol in mild persistent asthma. The OPTIMA Randomized Trial. Am J Respir Crit Care Med 2001; 164:1392–1397.
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994; 344:219–224.
- Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med 1996; 153:1481–1488.
- Walters EH, Walters JAE, Gibson MDP. Long-acting beta2-agonists for stable chronic asthma. Cochrane Database Syst Rev 2003; (3):CD001385. doi:10.1002/14651858.CD001385.
- Masoli M, Weatherall M, Holt S, Beasley R. Moderate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroid in symptomatic asthma. Thorax 2005; 60:730–734.
- Sin DD, Man J, Sharpe H, Gan WQ, Man SFP. Pharmacological management to reduce exacerbations in adults with asthma. A systematic review and meta-analysis. JAMA 2004; 292:367–376.
- Mann RD, Kubota K, Pearce G, Wilton L. Salmeterol: a study by prescription event monitoring in a UK cohort of 15,407 patients. J Clin Epidemiol 1996; 49:247–250.
- Lanes S, Lanza L, Wentworth C. Risk of emergency care, hospitalization, and ICU stays for acute asthma among recipients of salmeterol. Am J Respir Crit Care Med 1998; 158:857–861.
- Meier CR, Jick H. Drug use and pulmonary death rates in increasingly symptomatic asthma patients in the UK. Thorax 1997; 52:612–617.
- Williams C, Crossland L, Finnerty J, et al. A case control study of salmeterol and near-fatal attacks of asthma. Thorax 1998; 53:7–13.
- Lanes S, Garcia Rodriguez LA, Herta C. Respiratory medications and risk of asthma death. Thorax 2002; 57:683–686.
- Anderson HR, Ayres JG, Sturdy PM, et al. Bronchodilator treatment and deaths from asthma: case control study. Br Med J 2005; 330:117–124.
- Martinez FD. Safety of long-acting beta agonists—an urgent need to clear the air. N Engl J Med 2005; 353:2637–2639.
- Nelson HS. Long-acting beta-agonists in adult asthma: evidence that these drugs are safe. Prim Care Respir J 2006; 15:271–277.
- Lang DM. The long-acting beta agonist controversy: a critical examination of the evidence. Cleve Clin J Med 2006; 73:973–992.
- Rambasek T, Lang DM, Kavuru M. Omalizumab: where does it fit in current asthma management? Cleve Clin J Med 2004; 71:251–261.
- McGlashan D, Bochner B, Adelman D, et al. Down regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol 1997; 158:1438–1445.
- Busse W, Corren J, Lanier B, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001; 108:184–190.
- Soler M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces asthma exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001; 18:254–261.
- Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005; 60:309–316.
- Van Ganse E, Antonicelli L, Zhang Q, et al. Asthma-related resource use and cost by GINA classification of severity in three European countries. Respir Med 2006; 100:140–147.
- Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J 2002; 19:61–67.
- Cisternas MG, Blanc PH, Yen IH, et al. A comprehensive study of the direct and indirect costs of adult asthma. J Allergy Clin Immunol 2003; 111:1212–1218.
- Sullivan S, Turk F. An evaluation of the cost effectiveness of omalizumab for the treatment of severe persistent asthma. Allergy 2008; 63:670–684.
- US Food and Drug Administration. Omalizumab (marketed as Xolair) information. www.fda.gov/cder/drug/infopage/omalizumab/default.htm. Accessed August 31, 2007.
- Williams SG, Schmidt DK, Redd SC, Storms W. Key clinical activities for quality asthma care. Recommendations of the National Asthma Education and Prevention Program. MMWR Recomm Rep 2003; 52 RR-6:1–8.
- Cox L, Li J, Nelson H, Lockey R, et al. Allergy Immunotherapy: a practice parameter second update. J Allergy Clin Immunol 2007; 120:S25–S85.
- Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol 2007; 119:780–789.
- Murray AB, Milner RA. The accuracy of features in the clinical history for predicting atopic sensitization to airborne allergens in children. J Allergy Clin Immunol 1995; 96:588–596.
- Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100 suppl 3:1S–148S.
- Walker S, Pajno GB, Lima MT, Wilson DR, Durham SR. Grass pollen immunotherapy for seasonal rhinitis and asthma: a randomized, controlled trial. J Allergy Clin Immunol 2001; 107:87–93.
- Varney VA, Edwards J, Tabbah K, Brewster H, Mavroleon G, Frew AJ. Clinical efficacy of specific immunotherapy to cat dander: a double-blind placebo-controlled trial. Clin Exp Allergy 1997; 27:860–867.
- Cantani A, Arcese G, Lucenti P, Gagliesi D, Bartolucci M. A three-year prospective study of specific immunotherapy to inhalant allergens: evidence of safety and efficacy in 300 children with allergic asthma. J Investig Allergol Clin Immunol 1997; 7:90–97.
- Hedlin G, Wille S, Browaldh L, et al. Immunotherapy in children with allergic asthma: effect on bronchial hyperreactivity and pharmacotherapy. J Allergy Clin Immunol 1999; 103:609–614.
- Arvidsson MB, Löwhagen O, Rak S. Allergen specific immunotherapy attenuates early and late phase reactions in lower airways of birch pollen asthmatic patients: a double blind placebo-controlled study. Allergy 2004; 59:74–80.
- Pichler CE, Helbling A, Pichler WJ. Three years of specific immunotherapy with house-dust-mite extracts in patients with rhinitis and asthma: significant improvement of allergen-specific parameters and of nonspecific bronchial hyperreactivity. Allergy 2001; 56:301–306.
- Mirone C, Albert F, Tosi A, et al. Efficacy and safety of subcutaneous immunotherapy with a biologically standardized extract of Ambrosia artemisiifolia pollen: a double-blind, placebo-controlled study. Clin Exp Allergy 2004; 34:1408–1414.
- Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev 2003; (4):CD001186.
- Jacobsen L. Preventive aspects of immunotherapy: prevention for children at risk of developing asthma. Ann Allergy Asthma Immunol 2001; 87:43–46.
- Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT study). J Allergy Clin Immunol 2002; 109:251–256.
- Niggemann B, Jacobsen L, Dreborg S, et al; PAT Investigator Group. Five year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy 2006: 61:855–859.
- Des Roches A, Paradis L, Menardo JL, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract VI: specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol 1997; 99:450–453.
- Pajno GB, Barberio G, DeLuca F, et al. Prevention of new sensitizations in asthmatic children monosensitized to the house dust mite by specific immunotherapy: a six year follow up study. Clin Exp Allergy 2001; 31:1392–1397.
- Lang DM. Do beta blockers really enhance the risk of anaphylaxis during immunotherapy? Curr Allergy Asthma Rep 2008; 8:37–44.
- National Heart, Lung, and Blood institute, National Asthma education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. www.nhlbi.nih.gov/guidelines/asthma. Accessed 8/7/08.
- Expert Panel Report 2: Guidelines for the diagnosis and management of asthma. U.S. Department of Health and Human Services. Publication No. 97-4051; 1997.
- Expert Panel Report: Guidelines for the diagnosis and management of asthma. Update on Selected Topics—2002. J Allergy Clin Immunol 2002; 110:S141–S207.
- FitzGerald JM, Boulet LP, McIvor RA, Zimmerman S, Chapman KR. Asthma control in Canada remains suboptimal: the Reality of Asthma Control (TRAC) study. Can Respir J 2006; 13:253–259.
- Braganza S, Sharif I, Ozuah P. Documenting asthma severity: do we get it right? J Asthma 2003; 40:661–665.
- Cockcroft DW, Swystun VA. Asthma control versus asthma severity. J Allergy Clin Immunol 1996; 98:1016–1018.
- Peters SP, Jones CA, Haselkorn T, Mink DR, Valacer DJ, Weiss ST. Real-world Evaluation of Asthma Control and Treatment (REACT): findings from a national Web-based survey. J Allergy Clin Immunol. 2007; 119:1454–1461.
- Osborne ML, Vollmer WM, Pedula KL, Wilkins J, Buist AS, O’Hollaren M. Lack of correlation of symptoms with specialist-assessed long-term asthma severity. Chest 1999; 115:85–91.
- Li JT, Oppenheimer J, Bernstein IL, et al. Attaining optimal asthma control: a practice parameter. J Allergy Clin Immunol 2005; 116:S3–S11.
- Nathan RA, Sorkness C, Kosinski M, et al. Development of the Asthma Control Test: a survey for assessing asthma control. J Allergy Clin Immunol 2004; 113:59–65.
- Schatz M, Zeiger RS, Drane A, et al. Reliability and predictive validity of the Asthma Control Test administered by telephone calls using speech recognition technology. J Allergy Clin Immunol 2007; 119:336–343.
- Peters D, Chen C, Markson LE, Allen-Ramey FC, Vollmer WM. Using an asthma control questionnaire and administrative data to predict healthcare utilization. Chest 2006; 129:918–924.
- Schatz M, Sorkness C, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006; 117:549–556.
- Bateman E, Boushey H, Bousquet J, et al. Can guideline-defined asthma control be achieved? Am J Respir Crit Care Med 2004; 170:836–844.
- Davies TJ, Bunn WB, Fromer L, Gelfand EW, Colice GL. A focus on the asthma HEDIS measure and its implications for clinical practice. Manag Care Interface 2006; 19:29–36.
- Rubinfeld AR, Pain MC. Perception of asthma. Lancet 1976; 1:882–884.
- Teeter J, Bleecker E. Relationship between airway obstruction and respiratory symptoms in adult asthmatics. Chest 1998; 113:272–277.
- Shingo S, Zhang J, Reiss T. Correlation of airway obstruction and patient reported endpoints in clinical studies. Eur Resp J 2001; 17:220–224.
- Juniper EF, Bousquet J, Abetz L, Bateman ED; GOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med 2006; 100:616–621.
- Nelson H, Weiss S, Bleecker E, Yancey S, Dorinsky P. The Salmeterol Multicenter Asthma Research Trial. Chest 2006; 129:15–26.
- Wechsler M, Lehman E, Lazarus S, et al. ß-Adrenergic receptor polymorphisms and response to salmeterol. Am J Respir Crit Care Med 2006; 173:519–526.
- Palmer CNA, Lipworth BJ, Lee S, Ismail T, MacGregor DF, Mukhopadhyay S. Arginine-16 beta-2 adrenoceptor genotype predisposes to exacerbations in young asthmatics taking regular salmeterol. Thorax 2006; 61:940–944.
- Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of beta 2 adrenoceptor polymorphism. Thorax 2000; 55:762–727.
- Bleecker E, Postma D, Lawrance R, Meyers D, Ambrose H, Goldman M. Effect of ADRB2 polymorphisms on response to long-acting beta2-agonist therapy: a pharmacogenetic analysis of two randomized studies. Lancet 2007; 370:2118–2125.
- Bleecker E, Yancey S, Baitinger L, et al. Salmeterol response is not affected by beta-2 adrenergic receptor genotype in subjects with persistent asthma. J Allergy Clin Immunol 2006; 118:809–816.
- Nelson H, Bleecker E, Corren J, et al. Characterization of asthma exacerbations by Arg16Gly genotype in subjects with asthma receiving salmeterol alone or with fluticasone propionate. J Allergy Clin Immunol 2008; 121:S131.
- O’Byrne P, Barnes P, Rodriguez-Roisin R, et al. Low dose Inhaled budesonide and formoterol in mild persistent asthma. The OPTIMA Randomized Trial. Am J Respir Crit Care Med 2001; 164:1392–1397.
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994; 344:219–224.
- Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med 1996; 153:1481–1488.
- Walters EH, Walters JAE, Gibson MDP. Long-acting beta2-agonists for stable chronic asthma. Cochrane Database Syst Rev 2003; (3):CD001385. doi:10.1002/14651858.CD001385.
- Masoli M, Weatherall M, Holt S, Beasley R. Moderate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroid in symptomatic asthma. Thorax 2005; 60:730–734.
- Sin DD, Man J, Sharpe H, Gan WQ, Man SFP. Pharmacological management to reduce exacerbations in adults with asthma. A systematic review and meta-analysis. JAMA 2004; 292:367–376.
- Mann RD, Kubota K, Pearce G, Wilton L. Salmeterol: a study by prescription event monitoring in a UK cohort of 15,407 patients. J Clin Epidemiol 1996; 49:247–250.
- Lanes S, Lanza L, Wentworth C. Risk of emergency care, hospitalization, and ICU stays for acute asthma among recipients of salmeterol. Am J Respir Crit Care Med 1998; 158:857–861.
- Meier CR, Jick H. Drug use and pulmonary death rates in increasingly symptomatic asthma patients in the UK. Thorax 1997; 52:612–617.
- Williams C, Crossland L, Finnerty J, et al. A case control study of salmeterol and near-fatal attacks of asthma. Thorax 1998; 53:7–13.
- Lanes S, Garcia Rodriguez LA, Herta C. Respiratory medications and risk of asthma death. Thorax 2002; 57:683–686.
- Anderson HR, Ayres JG, Sturdy PM, et al. Bronchodilator treatment and deaths from asthma: case control study. Br Med J 2005; 330:117–124.
- Martinez FD. Safety of long-acting beta agonists—an urgent need to clear the air. N Engl J Med 2005; 353:2637–2639.
- Nelson HS. Long-acting beta-agonists in adult asthma: evidence that these drugs are safe. Prim Care Respir J 2006; 15:271–277.
- Lang DM. The long-acting beta agonist controversy: a critical examination of the evidence. Cleve Clin J Med 2006; 73:973–992.
- Rambasek T, Lang DM, Kavuru M. Omalizumab: where does it fit in current asthma management? Cleve Clin J Med 2004; 71:251–261.
- McGlashan D, Bochner B, Adelman D, et al. Down regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol 1997; 158:1438–1445.
- Busse W, Corren J, Lanier B, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001; 108:184–190.
- Soler M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces asthma exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001; 18:254–261.
- Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005; 60:309–316.
- Van Ganse E, Antonicelli L, Zhang Q, et al. Asthma-related resource use and cost by GINA classification of severity in three European countries. Respir Med 2006; 100:140–147.
- Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J 2002; 19:61–67.
- Cisternas MG, Blanc PH, Yen IH, et al. A comprehensive study of the direct and indirect costs of adult asthma. J Allergy Clin Immunol 2003; 111:1212–1218.
- Sullivan S, Turk F. An evaluation of the cost effectiveness of omalizumab for the treatment of severe persistent asthma. Allergy 2008; 63:670–684.
- US Food and Drug Administration. Omalizumab (marketed as Xolair) information. www.fda.gov/cder/drug/infopage/omalizumab/default.htm. Accessed August 31, 2007.
- Williams SG, Schmidt DK, Redd SC, Storms W. Key clinical activities for quality asthma care. Recommendations of the National Asthma Education and Prevention Program. MMWR Recomm Rep 2003; 52 RR-6:1–8.
- Cox L, Li J, Nelson H, Lockey R, et al. Allergy Immunotherapy: a practice parameter second update. J Allergy Clin Immunol 2007; 120:S25–S85.
- Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol 2007; 119:780–789.
- Murray AB, Milner RA. The accuracy of features in the clinical history for predicting atopic sensitization to airborne allergens in children. J Allergy Clin Immunol 1995; 96:588–596.
- Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100 suppl 3:1S–148S.
- Walker S, Pajno GB, Lima MT, Wilson DR, Durham SR. Grass pollen immunotherapy for seasonal rhinitis and asthma: a randomized, controlled trial. J Allergy Clin Immunol 2001; 107:87–93.
- Varney VA, Edwards J, Tabbah K, Brewster H, Mavroleon G, Frew AJ. Clinical efficacy of specific immunotherapy to cat dander: a double-blind placebo-controlled trial. Clin Exp Allergy 1997; 27:860–867.
- Cantani A, Arcese G, Lucenti P, Gagliesi D, Bartolucci M. A three-year prospective study of specific immunotherapy to inhalant allergens: evidence of safety and efficacy in 300 children with allergic asthma. J Investig Allergol Clin Immunol 1997; 7:90–97.
- Hedlin G, Wille S, Browaldh L, et al. Immunotherapy in children with allergic asthma: effect on bronchial hyperreactivity and pharmacotherapy. J Allergy Clin Immunol 1999; 103:609–614.
- Arvidsson MB, Löwhagen O, Rak S. Allergen specific immunotherapy attenuates early and late phase reactions in lower airways of birch pollen asthmatic patients: a double blind placebo-controlled study. Allergy 2004; 59:74–80.
- Pichler CE, Helbling A, Pichler WJ. Three years of specific immunotherapy with house-dust-mite extracts in patients with rhinitis and asthma: significant improvement of allergen-specific parameters and of nonspecific bronchial hyperreactivity. Allergy 2001; 56:301–306.
- Mirone C, Albert F, Tosi A, et al. Efficacy and safety of subcutaneous immunotherapy with a biologically standardized extract of Ambrosia artemisiifolia pollen: a double-blind, placebo-controlled study. Clin Exp Allergy 2004; 34:1408–1414.
- Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev 2003; (4):CD001186.
- Jacobsen L. Preventive aspects of immunotherapy: prevention for children at risk of developing asthma. Ann Allergy Asthma Immunol 2001; 87:43–46.
- Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT study). J Allergy Clin Immunol 2002; 109:251–256.
- Niggemann B, Jacobsen L, Dreborg S, et al; PAT Investigator Group. Five year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy 2006: 61:855–859.
- Des Roches A, Paradis L, Menardo JL, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract VI: specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol 1997; 99:450–453.
- Pajno GB, Barberio G, DeLuca F, et al. Prevention of new sensitizations in asthmatic children monosensitized to the house dust mite by specific immunotherapy: a six year follow up study. Clin Exp Allergy 2001; 31:1392–1397.
- Lang DM. Do beta blockers really enhance the risk of anaphylaxis during immunotherapy? Curr Allergy Asthma Rep 2008; 8:37–44.
KEY POINTS
- The EPR3 recommends that management decisions be based initially on asthma severity, and subsequently on asthma control as assessed serially by validated tests.
- Omalizumab, a monoclonal antibody against immunoglobulin E, is the only adjunctive therapy to demonstrate efficacy when added to high-dose inhaled corticosteroids plus long-acting beta agonists in patients with severe, persistent, allergic asthma.
- The EPR3 guidelines recommend consideration of allergen immunotherapy for patients with mild or moderate persistent allergic asthma.