User login
Oxytocin and prostaglandins are critically important regulators of uterine contraction. Obstetrician-gynecologists commonly prescribe oxytocin and prostaglandin agonists (misoprostol, dinoprostone) to stimulate uterine contraction for the induction of labor, prevention and treatment of postpartum hemorrhage, and treatment of miscarriage and fetal demise. The focus of this editorial is the clinical pharmacology of misoprostol.
Misoprostol is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of nonsteroidal anti-inflammatory drug–induced gastric ulcers and for patients at high risk for gastric ulcers, including those with a history of gastric ulcers. The approved misoprostol route and dose for this indication is oral administration of 200 µg four times daily with food.1 Recent food intake and antacid use reduces the absorption of orally administered misoprostol. There are no FDA-approved indications for the use of misoprostol as a single agent in obstetrics and gynecology. The FDA has approved the combination of mifepristone and misoprostol for medication abortion in the first trimester. In contrast to misoprostol, PGE2 (dinoprostone) is approved by the FDA as a vaginal insert containing 10 mg of dinoprostone for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetric indication for induction of labor (Cervidil; Ferring Pharmaceuticals Inc, Parsippany, New Jersey).2
Pharmacology of misoprostol
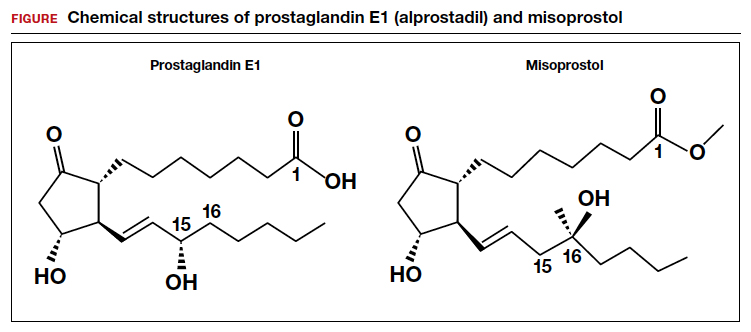
Misoprostol is a prostaglandin E1 (PGE1) agonist analogue. Prostaglandin E1 (alprostadil) is rapidly metabolized, has a half-life in the range of minutes and is not orally active, requiring administration by intravenous infusion or injection. It is indicated to maintain a patent ductus arteriosus in newborns with ductal-dependent circulation and to treat erectile dysfunction.3 In contrast to PGE1, misoprostol has a methyl ester group at carbon-1 (C-1) that increases potency and duration of action. Misoprostol also has no hydroxyl group at C-15, replacing that moiety with the addition of both a methyl- and hydroxyl- group at C-16 (FIGURE). These molecular changes improve oral activity and increase duration of action.4 Pure misoprostol is a viscous oil. It is formulated into tables by dispersing the oil on hydroxypropyl methyl cellulose before compounding into tablets. Unlike naturally occurring prostaglandins (PGE1), misoprostol tablets are stabile at room temperature for years.4
Following absorption, the methyl ester at C-1 is enzymatically cleaved, yielding misoprostol acid, the active drug.4 Misoprostol binds to the E prostanoid receptor 3 (EP-3).5 Activation of myometrial EP-3 receptor induces an increase in intracellular phosphoinositol turnover and calcium mobilization, resulting in an increase in intracellular-free calcium, triggering actin-myosin contractility.6 The increase in free calcium is propagated cell-to-cell through gap junctions that link the myometrial cells to facilitate the generation of a coordinated contraction.
Misoprostol: Various routes of administration are not equal
Misoprostol can be given by an oral, buccal, vaginal, or rectal route of administration. To study the effect of the route of administration on uterine tone and contractility, investigators randomly assigned patients at 8 to 11 weeks’ gestation to receive misoprostol 400 µg as a single dose by the oral or vaginal route. Uterine tone and contractility were measured using an intrauterine pressure transducer. Compared to vaginal administration, oral administration of misprostol was associated with rapid attainment of peak plasma level at 30 minutes, followed by a decline in concentration by 60 minutes. This rapid onset and rapid offset of plasma concentration was paralleled by the onset of uterine tone within 8 minutes, but surprisingly no sustained uterine contractions.7 By contrast, following vaginal administration of misoprostol, serum levels rose slowly and peaked in 1 to 2 hours. Uterine tone increased within 21 minutes, and sustained uterine contractions were recorded for 4 hours.7 The rapid rise and fall in plasma misoprostol following oral administration and the more sustained plasma misoprostol concentration over 4 hours has been previously reported.8 In a second study involving patients 8 to 11 weeks’ gestation, the effect of a single dose of misoprostol 400 µg by an oral or vaginal route on uterine contractility was compared using an intrauterine pressure transducer.9 Confirming previous results, the time from misoprostol administration to increased uterine tone was more rapid with oral than with vaginal administration (8 min vs 19 min). Over the course of 4 hours, uterine contraction activity was greater with vaginal than with oral administration (454 vs 166 Montevideo units).9
Both studies reported that oral administration of misoprostol resulted in more rapid onset and offset of action than vaginal administration. Oral administration of a single dose of misoprostol 400 µg did not result in sustained uterine contractions in most patients in the first trimester. Vaginal administration produced a slower onset of increased uterine tone but sustained uterine contractions over 4 hours. Compared with vaginal administration of misoprostol, the rapid onset and offset of action of oral misoprostol may reduce the rate of tachysystole and changes in fetal heart rate observed with vaginal administration.10
An important finding is that buccal and vaginal administration of misoprostol have similar effects on uterine tone in the first trimester.11 To study the effect of buccal and vaginal administration of misoprostol on uterine tone, patients 6 to 13 weeks’ gestation were randomly allocated to receive a single dose of misoprostol 400 µg by a buccal or vaginal route.11 Uterine activity over 5 hours following administration was assessed using an intrauterine pressure transducer. Uterine tone 20 to 30 minutes after buccal or vaginal administration of misoprostol (400 µg) was 27 and 28 mm Hg, respectively. Peak uterine tone, as measured by an intrauterine pressure transducer, for buccal and vaginal administration of misoprostol was 49 mm Hg and 54 mm Hg, respectively. Total Alexandria units (AU) over 5 hours following buccal or vaginal administration was 6,537 AU and 6,090 AU, respectively.11
An AU is calculated as the average amplitude of the contractions (mm Hg) multiplied by the average duration of the contractions (min) multiplied by average frequency of contraction over 10 minutes.12 By contrast, a Montevideo unit does not include an assessment of contraction duration and is calculated as average amplitude of contractions (mm Hg) multiplied by frequency of uterine contractions over 10 minutes.12
In contrast to buccal or vaginal administration, rectal administration of misoprostol resulted in much lower peak uterine tone and contractility as measured by a pressure transducer. Uterine tone 20 to 30 minutes after vaginal and rectal administration of misoprostol (400 µg) was 28 and 19 mm Hg, respectively.11 Peak uterine tone, as measured by an intrauterine pressure transducer, for vaginal and rectal administration of misoprostol was 54 and 31 mm Hg, respectively. AUs over 5 hours following vaginal and rectal administration was 6,090 AU and 2,768 AU, respectively.11 Compared with buccal and vaginal administration of misoprostol, rectal administration produced less sustained uterine contractions in the first trimester of pregnancy. To achieve maximal sustained uterine contractions, buccal and vaginal routes of administration are superior to oral and rectal administration.
Continue to: Misoprostol and cervical ripening...
Misoprostol and cervical ripening
Misoprostol is commonly used to soften and ripen the cervix. Some of the cervical ripening effects of misoprostol are likely due to increased uterine tone. In addition, misoprostol may have a direct effect on the collagen structure of the cervix. To study the effect of misoprostol on the cervix, pregnant patients in the first trimester were randomly assigned to receive misoprostol 200 µg by vaginal self-administration, isosorbide mononitrate (IMN) 40 mg by vaginal self-administration or no treatment the evening prior to pregnancy termination.13 The following day, before uterine evacuation, a cervical biopsy was obtained for electron microscopy studies and immunohistochemistry to assess the presence of enzymes involved in collagen degradation, including matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 9 (MMP-9). Electron microscopy demonstrated that pretreatment with misoprostol resulted in a pronounced splitting and disorganization of collagen fibers.13 Compared with misoprostol treatment, IMN produced less splitting and disorganization of collagen fibers, and in the no treatment group, no marked changes in the collagen framework were observed.
Compared with no treatment, misoprostol and IMN pretreatment were associated with marked increases in MMP-1 and MMP-9 as assessed by immunohistochemistry. Misoprostol pretreatment also resulted in a significant increase in interleukin-8 concentration compared with IMN pretreatment and no treatment (8.8 vs 2.7 vs 2.4 pg/mg tissue), respectively.13 Other investigators have also reported that misoprostol increased cervical leukocyte influx and collagen disrupting enzymes MMP-8 and MMP-9.14,15
An open-label clinical trial compared the efficacy of misoprostol versus Foley catheter for labor induction at term in 1,859 patients ≥ 37 weeks’ gestation with a Bishop score <6.16 Patients were randomly allocated to misoprostol (50 µg orally every 4 hours up to 3 times in 24 hours) versus placement of a 16 F or 18 F Foley catheter introduced through the cervix, filled with 30 mL of sodium chloride or water. The investigators reported that oral misoprostol and Foley catheter cervical ripening had similar safety and effectiveness for cervical ripening as a prelude to induction of labor, including no statistically significant differences in 5-minute Apgar score <7, umbilical cord artery pH ≤ 7.05, postpartum hemorrhage, or cesarean birth rate.16
Bottom line
Misoprostol and oxytocin are commonly prescribed in obstetric practice for cervical ripening and induction of labor, respectively. The dose and route of administration of misoprostol influences the effect on the uterus. For cervical ripening, where rapid onset and offset may help to reduce the risk of uterine tachysystole and worrisome fetal heart rate changes, low-dose (50 µg) oral administration of misoprostol may be a preferred dose and route. For the treatment of miscarriage and fetal demise, to stimulate sustained uterine contractions over many hours, buccal and vaginal administration of misoprostol are preferred. Rectal administration is generally inferior to buccal and vaginal administration for stimulating sustained uterine contractions and its uses should be limited. ●
Common side effects of misoprostol are abdominal cramping, diarrhea, nausea, vomiting, headache, and fever. Elevated temperature following misoprostol administration is a concerning side effect that may require further investigation to rule out an infection, especially if the elevated temperature persists for > 4 hours. The preoptic area of the anterior hypothalamus (POAH) plays a major role in thermoregulation. When an infection causes an increase in endogenous pyrogens, including interleukin-1β, interleukin-6 and tumor necrosis factor, prostaglandins are generated in the region of the POAH, increasing the thermoregulatory set point, triggering cutaneous vasoconstriction and shivering and non-shivering thermogenesis.1 Misoprostol, especially at doses >400 µg commonly causes both patient-reported chills and temperature elevation >38° C.
In a study comparing misoprostol and oxytocin for the management of the third stage of labor, 597 patients were randomly allocated to receive oxytocin 10 units by intramuscular injection or misoprostol 400 µg or 600 µg by the oral route.2 Patient-reported shivering occurred in 13%, 19%, and 28% of patients receiving oxytocin, misoprostol 400 µg and misoprostol 800 µg, respectively. A recorded temperature >38° C occurred within 1 hour of medication administration in approximately 3%, 2%, and 7.5% of patients receiving oxytocin, misoprostol 400 µg, and misoprostol 800 µg, respectively. In another study, 453 patients scheduled for a cesarean birth were randomly allocated to receive 1 of 3 doses of rectal misoprostol 200 μg, 400 μg, or 600 μg before incision. Fever was detected in 2.6%, 9.9%, and 5.1% of the patients receiving misoprostol 200 μg, 400 μg, or 600 μg, respectively.3
References
1. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304-315. doi: 10.1016/s0002-9343(01)00834-8.
2. Lumbiganon P, Hofmeyr J, Gumezoglu AM, et al. Misoprostol dose-related shivering and pyrexia in the third stage of labor. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labor. Br J Obstet Gynaecol. 1999;106:304-308. doi: 10.1111/j.1471-0528.1999.tb08266.x.
3. Sweed M, El-Said M, Abou-Gamrah AA, et al. Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarean section: a randomized clinical trial. J Obstet Gynaecol Res. 2019;45:585-591. doi: 10.1111 /jog.13883.
- Cytotec [package insert]. Chicago, IL: GD Searle & Co. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf. Accessed June 20, 2022.
- Cervidil [package insert]. St Louis, MO: Forrest Pharmaceuticals Inc.; May 2006. Accessed June 20, 2022.
- Caverject [package insert]. New York, NY: Pfizer Inc.; March 2014. Accessed June 20, 2022.
- Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev. 1990;10:149-172. doi: 10.1002/med.2610100202.
- Audit M, White KI, Breton B, et al. Crystal structure of misoprostol bound to the labor inducer prostaglandin E2 receptor. Nat Chem Biol. 2019;15:11-17. doi: 10.1038/s41589-018-0160-y.
- Pallliser KH, Hirst JJ, Ooi G, et al. Prostaglandin E and F receptor expression and myometrial sensitivity in labor onset in the sheep. Biol Reprod. 2005;72:937-943. doi: 10.1095/biolreprod.104.035311.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280. doi: 10.1016/s0029-7844(98)00436-0.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92. doi: 10.1016/S0029-7844(97)00111-7.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84. doi: 10.1093/humrep/deh005.
- Young DC, Delaney T, Armson BA, et al. Oral misoprostol, low dose vaginal misoprostol and vaginal dinoprostone for labor induction: randomized controlled trial. PLOS One. 2020;15:e0227245. doi: 10.1371/journal.pone.0227245.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01.AOG.0000230398.32794.9d.
- el-Sahwi S, Gaafar AA, Toppozada HK. A new unit for evaluation of uterine activity. Am J Obstet Gynecol. 1967;98:900-903. doi: 10.1016/0002-9378(67)90074-9.
- Vukas N, Ekerhovd E, Abrahamsson G, et al. Cervical priming in the first trimester: morphological and biochemical effects of misoprostol and isosorbide mononitrate. Acta Obstet Gyecol. 2009;88:43-51. doi: 10.1080/00016340802585440.
- Aronsson A, Ulfgren AK, Stabi B, et al. The effect of orally and vaginally administered misoprostol on inflammatory mediators and cervical ripening during early pregnancy. Contraception. 2005;72:33-39. doi: 10.1016/j.contraception.2005.02.012.
- Denison FC, Riley SC, Elliott CL, et al. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6:541-548. doi: 10.1093/molehr/6.6.541.
- ten Eikelder MLG, Rengerink KO, Jozwiak M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet. 2016;387:1619-1628. doi: 10.1016 /S0140-6736(16)00084-2.
Oxytocin and prostaglandins are critically important regulators of uterine contraction. Obstetrician-gynecologists commonly prescribe oxytocin and prostaglandin agonists (misoprostol, dinoprostone) to stimulate uterine contraction for the induction of labor, prevention and treatment of postpartum hemorrhage, and treatment of miscarriage and fetal demise. The focus of this editorial is the clinical pharmacology of misoprostol.
Misoprostol is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of nonsteroidal anti-inflammatory drug–induced gastric ulcers and for patients at high risk for gastric ulcers, including those with a history of gastric ulcers. The approved misoprostol route and dose for this indication is oral administration of 200 µg four times daily with food.1 Recent food intake and antacid use reduces the absorption of orally administered misoprostol. There are no FDA-approved indications for the use of misoprostol as a single agent in obstetrics and gynecology. The FDA has approved the combination of mifepristone and misoprostol for medication abortion in the first trimester. In contrast to misoprostol, PGE2 (dinoprostone) is approved by the FDA as a vaginal insert containing 10 mg of dinoprostone for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetric indication for induction of labor (Cervidil; Ferring Pharmaceuticals Inc, Parsippany, New Jersey).2
Pharmacology of misoprostol
Misoprostol is a prostaglandin E1 (PGE1) agonist analogue. Prostaglandin E1 (alprostadil) is rapidly metabolized, has a half-life in the range of minutes and is not orally active, requiring administration by intravenous infusion or injection. It is indicated to maintain a patent ductus arteriosus in newborns with ductal-dependent circulation and to treat erectile dysfunction.3 In contrast to PGE1, misoprostol has a methyl ester group at carbon-1 (C-1) that increases potency and duration of action. Misoprostol also has no hydroxyl group at C-15, replacing that moiety with the addition of both a methyl- and hydroxyl- group at C-16 (FIGURE). These molecular changes improve oral activity and increase duration of action.4 Pure misoprostol is a viscous oil. It is formulated into tables by dispersing the oil on hydroxypropyl methyl cellulose before compounding into tablets. Unlike naturally occurring prostaglandins (PGE1), misoprostol tablets are stabile at room temperature for years.4
Following absorption, the methyl ester at C-1 is enzymatically cleaved, yielding misoprostol acid, the active drug.4 Misoprostol binds to the E prostanoid receptor 3 (EP-3).5 Activation of myometrial EP-3 receptor induces an increase in intracellular phosphoinositol turnover and calcium mobilization, resulting in an increase in intracellular-free calcium, triggering actin-myosin contractility.6 The increase in free calcium is propagated cell-to-cell through gap junctions that link the myometrial cells to facilitate the generation of a coordinated contraction.

Misoprostol: Various routes of administration are not equal
Misoprostol can be given by an oral, buccal, vaginal, or rectal route of administration. To study the effect of the route of administration on uterine tone and contractility, investigators randomly assigned patients at 8 to 11 weeks’ gestation to receive misoprostol 400 µg as a single dose by the oral or vaginal route. Uterine tone and contractility were measured using an intrauterine pressure transducer. Compared to vaginal administration, oral administration of misprostol was associated with rapid attainment of peak plasma level at 30 minutes, followed by a decline in concentration by 60 minutes. This rapid onset and rapid offset of plasma concentration was paralleled by the onset of uterine tone within 8 minutes, but surprisingly no sustained uterine contractions.7 By contrast, following vaginal administration of misoprostol, serum levels rose slowly and peaked in 1 to 2 hours. Uterine tone increased within 21 minutes, and sustained uterine contractions were recorded for 4 hours.7 The rapid rise and fall in plasma misoprostol following oral administration and the more sustained plasma misoprostol concentration over 4 hours has been previously reported.8 In a second study involving patients 8 to 11 weeks’ gestation, the effect of a single dose of misoprostol 400 µg by an oral or vaginal route on uterine contractility was compared using an intrauterine pressure transducer.9 Confirming previous results, the time from misoprostol administration to increased uterine tone was more rapid with oral than with vaginal administration (8 min vs 19 min). Over the course of 4 hours, uterine contraction activity was greater with vaginal than with oral administration (454 vs 166 Montevideo units).9
Both studies reported that oral administration of misoprostol resulted in more rapid onset and offset of action than vaginal administration. Oral administration of a single dose of misoprostol 400 µg did not result in sustained uterine contractions in most patients in the first trimester. Vaginal administration produced a slower onset of increased uterine tone but sustained uterine contractions over 4 hours. Compared with vaginal administration of misoprostol, the rapid onset and offset of action of oral misoprostol may reduce the rate of tachysystole and changes in fetal heart rate observed with vaginal administration.10
An important finding is that buccal and vaginal administration of misoprostol have similar effects on uterine tone in the first trimester.11 To study the effect of buccal and vaginal administration of misoprostol on uterine tone, patients 6 to 13 weeks’ gestation were randomly allocated to receive a single dose of misoprostol 400 µg by a buccal or vaginal route.11 Uterine activity over 5 hours following administration was assessed using an intrauterine pressure transducer. Uterine tone 20 to 30 minutes after buccal or vaginal administration of misoprostol (400 µg) was 27 and 28 mm Hg, respectively. Peak uterine tone, as measured by an intrauterine pressure transducer, for buccal and vaginal administration of misoprostol was 49 mm Hg and 54 mm Hg, respectively. Total Alexandria units (AU) over 5 hours following buccal or vaginal administration was 6,537 AU and 6,090 AU, respectively.11
An AU is calculated as the average amplitude of the contractions (mm Hg) multiplied by the average duration of the contractions (min) multiplied by average frequency of contraction over 10 minutes.12 By contrast, a Montevideo unit does not include an assessment of contraction duration and is calculated as average amplitude of contractions (mm Hg) multiplied by frequency of uterine contractions over 10 minutes.12
In contrast to buccal or vaginal administration, rectal administration of misoprostol resulted in much lower peak uterine tone and contractility as measured by a pressure transducer. Uterine tone 20 to 30 minutes after vaginal and rectal administration of misoprostol (400 µg) was 28 and 19 mm Hg, respectively.11 Peak uterine tone, as measured by an intrauterine pressure transducer, for vaginal and rectal administration of misoprostol was 54 and 31 mm Hg, respectively. AUs over 5 hours following vaginal and rectal administration was 6,090 AU and 2,768 AU, respectively.11 Compared with buccal and vaginal administration of misoprostol, rectal administration produced less sustained uterine contractions in the first trimester of pregnancy. To achieve maximal sustained uterine contractions, buccal and vaginal routes of administration are superior to oral and rectal administration.
Continue to: Misoprostol and cervical ripening...
Misoprostol and cervical ripening
Misoprostol is commonly used to soften and ripen the cervix. Some of the cervical ripening effects of misoprostol are likely due to increased uterine tone. In addition, misoprostol may have a direct effect on the collagen structure of the cervix. To study the effect of misoprostol on the cervix, pregnant patients in the first trimester were randomly assigned to receive misoprostol 200 µg by vaginal self-administration, isosorbide mononitrate (IMN) 40 mg by vaginal self-administration or no treatment the evening prior to pregnancy termination.13 The following day, before uterine evacuation, a cervical biopsy was obtained for electron microscopy studies and immunohistochemistry to assess the presence of enzymes involved in collagen degradation, including matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 9 (MMP-9). Electron microscopy demonstrated that pretreatment with misoprostol resulted in a pronounced splitting and disorganization of collagen fibers.13 Compared with misoprostol treatment, IMN produced less splitting and disorganization of collagen fibers, and in the no treatment group, no marked changes in the collagen framework were observed.
Compared with no treatment, misoprostol and IMN pretreatment were associated with marked increases in MMP-1 and MMP-9 as assessed by immunohistochemistry. Misoprostol pretreatment also resulted in a significant increase in interleukin-8 concentration compared with IMN pretreatment and no treatment (8.8 vs 2.7 vs 2.4 pg/mg tissue), respectively.13 Other investigators have also reported that misoprostol increased cervical leukocyte influx and collagen disrupting enzymes MMP-8 and MMP-9.14,15
An open-label clinical trial compared the efficacy of misoprostol versus Foley catheter for labor induction at term in 1,859 patients ≥ 37 weeks’ gestation with a Bishop score <6.16 Patients were randomly allocated to misoprostol (50 µg orally every 4 hours up to 3 times in 24 hours) versus placement of a 16 F or 18 F Foley catheter introduced through the cervix, filled with 30 mL of sodium chloride or water. The investigators reported that oral misoprostol and Foley catheter cervical ripening had similar safety and effectiveness for cervical ripening as a prelude to induction of labor, including no statistically significant differences in 5-minute Apgar score <7, umbilical cord artery pH ≤ 7.05, postpartum hemorrhage, or cesarean birth rate.16
Bottom line
Misoprostol and oxytocin are commonly prescribed in obstetric practice for cervical ripening and induction of labor, respectively. The dose and route of administration of misoprostol influences the effect on the uterus. For cervical ripening, where rapid onset and offset may help to reduce the risk of uterine tachysystole and worrisome fetal heart rate changes, low-dose (50 µg) oral administration of misoprostol may be a preferred dose and route. For the treatment of miscarriage and fetal demise, to stimulate sustained uterine contractions over many hours, buccal and vaginal administration of misoprostol are preferred. Rectal administration is generally inferior to buccal and vaginal administration for stimulating sustained uterine contractions and its uses should be limited. ●
Common side effects of misoprostol are abdominal cramping, diarrhea, nausea, vomiting, headache, and fever. Elevated temperature following misoprostol administration is a concerning side effect that may require further investigation to rule out an infection, especially if the elevated temperature persists for > 4 hours. The preoptic area of the anterior hypothalamus (POAH) plays a major role in thermoregulation. When an infection causes an increase in endogenous pyrogens, including interleukin-1β, interleukin-6 and tumor necrosis factor, prostaglandins are generated in the region of the POAH, increasing the thermoregulatory set point, triggering cutaneous vasoconstriction and shivering and non-shivering thermogenesis.1 Misoprostol, especially at doses >400 µg commonly causes both patient-reported chills and temperature elevation >38° C.
In a study comparing misoprostol and oxytocin for the management of the third stage of labor, 597 patients were randomly allocated to receive oxytocin 10 units by intramuscular injection or misoprostol 400 µg or 600 µg by the oral route.2 Patient-reported shivering occurred in 13%, 19%, and 28% of patients receiving oxytocin, misoprostol 400 µg and misoprostol 800 µg, respectively. A recorded temperature >38° C occurred within 1 hour of medication administration in approximately 3%, 2%, and 7.5% of patients receiving oxytocin, misoprostol 400 µg, and misoprostol 800 µg, respectively. In another study, 453 patients scheduled for a cesarean birth were randomly allocated to receive 1 of 3 doses of rectal misoprostol 200 μg, 400 μg, or 600 μg before incision. Fever was detected in 2.6%, 9.9%, and 5.1% of the patients receiving misoprostol 200 μg, 400 μg, or 600 μg, respectively.3
References
1. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304-315. doi: 10.1016/s0002-9343(01)00834-8.
2. Lumbiganon P, Hofmeyr J, Gumezoglu AM, et al. Misoprostol dose-related shivering and pyrexia in the third stage of labor. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labor. Br J Obstet Gynaecol. 1999;106:304-308. doi: 10.1111/j.1471-0528.1999.tb08266.x.
3. Sweed M, El-Said M, Abou-Gamrah AA, et al. Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarean section: a randomized clinical trial. J Obstet Gynaecol Res. 2019;45:585-591. doi: 10.1111 /jog.13883.
Oxytocin and prostaglandins are critically important regulators of uterine contraction. Obstetrician-gynecologists commonly prescribe oxytocin and prostaglandin agonists (misoprostol, dinoprostone) to stimulate uterine contraction for the induction of labor, prevention and treatment of postpartum hemorrhage, and treatment of miscarriage and fetal demise. The focus of this editorial is the clinical pharmacology of misoprostol.
Misoprostol is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of nonsteroidal anti-inflammatory drug–induced gastric ulcers and for patients at high risk for gastric ulcers, including those with a history of gastric ulcers. The approved misoprostol route and dose for this indication is oral administration of 200 µg four times daily with food.1 Recent food intake and antacid use reduces the absorption of orally administered misoprostol. There are no FDA-approved indications for the use of misoprostol as a single agent in obstetrics and gynecology. The FDA has approved the combination of mifepristone and misoprostol for medication abortion in the first trimester. In contrast to misoprostol, PGE2 (dinoprostone) is approved by the FDA as a vaginal insert containing 10 mg of dinoprostone for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetric indication for induction of labor (Cervidil; Ferring Pharmaceuticals Inc, Parsippany, New Jersey).2
Pharmacology of misoprostol
Misoprostol is a prostaglandin E1 (PGE1) agonist analogue. Prostaglandin E1 (alprostadil) is rapidly metabolized, has a half-life in the range of minutes and is not orally active, requiring administration by intravenous infusion or injection. It is indicated to maintain a patent ductus arteriosus in newborns with ductal-dependent circulation and to treat erectile dysfunction.3 In contrast to PGE1, misoprostol has a methyl ester group at carbon-1 (C-1) that increases potency and duration of action. Misoprostol also has no hydroxyl group at C-15, replacing that moiety with the addition of both a methyl- and hydroxyl- group at C-16 (FIGURE). These molecular changes improve oral activity and increase duration of action.4 Pure misoprostol is a viscous oil. It is formulated into tables by dispersing the oil on hydroxypropyl methyl cellulose before compounding into tablets. Unlike naturally occurring prostaglandins (PGE1), misoprostol tablets are stabile at room temperature for years.4
Following absorption, the methyl ester at C-1 is enzymatically cleaved, yielding misoprostol acid, the active drug.4 Misoprostol binds to the E prostanoid receptor 3 (EP-3).5 Activation of myometrial EP-3 receptor induces an increase in intracellular phosphoinositol turnover and calcium mobilization, resulting in an increase in intracellular-free calcium, triggering actin-myosin contractility.6 The increase in free calcium is propagated cell-to-cell through gap junctions that link the myometrial cells to facilitate the generation of a coordinated contraction.

Misoprostol: Various routes of administration are not equal
Misoprostol can be given by an oral, buccal, vaginal, or rectal route of administration. To study the effect of the route of administration on uterine tone and contractility, investigators randomly assigned patients at 8 to 11 weeks’ gestation to receive misoprostol 400 µg as a single dose by the oral or vaginal route. Uterine tone and contractility were measured using an intrauterine pressure transducer. Compared to vaginal administration, oral administration of misprostol was associated with rapid attainment of peak plasma level at 30 minutes, followed by a decline in concentration by 60 minutes. This rapid onset and rapid offset of plasma concentration was paralleled by the onset of uterine tone within 8 minutes, but surprisingly no sustained uterine contractions.7 By contrast, following vaginal administration of misoprostol, serum levels rose slowly and peaked in 1 to 2 hours. Uterine tone increased within 21 minutes, and sustained uterine contractions were recorded for 4 hours.7 The rapid rise and fall in plasma misoprostol following oral administration and the more sustained plasma misoprostol concentration over 4 hours has been previously reported.8 In a second study involving patients 8 to 11 weeks’ gestation, the effect of a single dose of misoprostol 400 µg by an oral or vaginal route on uterine contractility was compared using an intrauterine pressure transducer.9 Confirming previous results, the time from misoprostol administration to increased uterine tone was more rapid with oral than with vaginal administration (8 min vs 19 min). Over the course of 4 hours, uterine contraction activity was greater with vaginal than with oral administration (454 vs 166 Montevideo units).9
Both studies reported that oral administration of misoprostol resulted in more rapid onset and offset of action than vaginal administration. Oral administration of a single dose of misoprostol 400 µg did not result in sustained uterine contractions in most patients in the first trimester. Vaginal administration produced a slower onset of increased uterine tone but sustained uterine contractions over 4 hours. Compared with vaginal administration of misoprostol, the rapid onset and offset of action of oral misoprostol may reduce the rate of tachysystole and changes in fetal heart rate observed with vaginal administration.10
An important finding is that buccal and vaginal administration of misoprostol have similar effects on uterine tone in the first trimester.11 To study the effect of buccal and vaginal administration of misoprostol on uterine tone, patients 6 to 13 weeks’ gestation were randomly allocated to receive a single dose of misoprostol 400 µg by a buccal or vaginal route.11 Uterine activity over 5 hours following administration was assessed using an intrauterine pressure transducer. Uterine tone 20 to 30 minutes after buccal or vaginal administration of misoprostol (400 µg) was 27 and 28 mm Hg, respectively. Peak uterine tone, as measured by an intrauterine pressure transducer, for buccal and vaginal administration of misoprostol was 49 mm Hg and 54 mm Hg, respectively. Total Alexandria units (AU) over 5 hours following buccal or vaginal administration was 6,537 AU and 6,090 AU, respectively.11
An AU is calculated as the average amplitude of the contractions (mm Hg) multiplied by the average duration of the contractions (min) multiplied by average frequency of contraction over 10 minutes.12 By contrast, a Montevideo unit does not include an assessment of contraction duration and is calculated as average amplitude of contractions (mm Hg) multiplied by frequency of uterine contractions over 10 minutes.12
In contrast to buccal or vaginal administration, rectal administration of misoprostol resulted in much lower peak uterine tone and contractility as measured by a pressure transducer. Uterine tone 20 to 30 minutes after vaginal and rectal administration of misoprostol (400 µg) was 28 and 19 mm Hg, respectively.11 Peak uterine tone, as measured by an intrauterine pressure transducer, for vaginal and rectal administration of misoprostol was 54 and 31 mm Hg, respectively. AUs over 5 hours following vaginal and rectal administration was 6,090 AU and 2,768 AU, respectively.11 Compared with buccal and vaginal administration of misoprostol, rectal administration produced less sustained uterine contractions in the first trimester of pregnancy. To achieve maximal sustained uterine contractions, buccal and vaginal routes of administration are superior to oral and rectal administration.
Continue to: Misoprostol and cervical ripening...
Misoprostol and cervical ripening
Misoprostol is commonly used to soften and ripen the cervix. Some of the cervical ripening effects of misoprostol are likely due to increased uterine tone. In addition, misoprostol may have a direct effect on the collagen structure of the cervix. To study the effect of misoprostol on the cervix, pregnant patients in the first trimester were randomly assigned to receive misoprostol 200 µg by vaginal self-administration, isosorbide mononitrate (IMN) 40 mg by vaginal self-administration or no treatment the evening prior to pregnancy termination.13 The following day, before uterine evacuation, a cervical biopsy was obtained for electron microscopy studies and immunohistochemistry to assess the presence of enzymes involved in collagen degradation, including matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 9 (MMP-9). Electron microscopy demonstrated that pretreatment with misoprostol resulted in a pronounced splitting and disorganization of collagen fibers.13 Compared with misoprostol treatment, IMN produced less splitting and disorganization of collagen fibers, and in the no treatment group, no marked changes in the collagen framework were observed.
Compared with no treatment, misoprostol and IMN pretreatment were associated with marked increases in MMP-1 and MMP-9 as assessed by immunohistochemistry. Misoprostol pretreatment also resulted in a significant increase in interleukin-8 concentration compared with IMN pretreatment and no treatment (8.8 vs 2.7 vs 2.4 pg/mg tissue), respectively.13 Other investigators have also reported that misoprostol increased cervical leukocyte influx and collagen disrupting enzymes MMP-8 and MMP-9.14,15
An open-label clinical trial compared the efficacy of misoprostol versus Foley catheter for labor induction at term in 1,859 patients ≥ 37 weeks’ gestation with a Bishop score <6.16 Patients were randomly allocated to misoprostol (50 µg orally every 4 hours up to 3 times in 24 hours) versus placement of a 16 F or 18 F Foley catheter introduced through the cervix, filled with 30 mL of sodium chloride or water. The investigators reported that oral misoprostol and Foley catheter cervical ripening had similar safety and effectiveness for cervical ripening as a prelude to induction of labor, including no statistically significant differences in 5-minute Apgar score <7, umbilical cord artery pH ≤ 7.05, postpartum hemorrhage, or cesarean birth rate.16
Bottom line
Misoprostol and oxytocin are commonly prescribed in obstetric practice for cervical ripening and induction of labor, respectively. The dose and route of administration of misoprostol influences the effect on the uterus. For cervical ripening, where rapid onset and offset may help to reduce the risk of uterine tachysystole and worrisome fetal heart rate changes, low-dose (50 µg) oral administration of misoprostol may be a preferred dose and route. For the treatment of miscarriage and fetal demise, to stimulate sustained uterine contractions over many hours, buccal and vaginal administration of misoprostol are preferred. Rectal administration is generally inferior to buccal and vaginal administration for stimulating sustained uterine contractions and its uses should be limited. ●
Common side effects of misoprostol are abdominal cramping, diarrhea, nausea, vomiting, headache, and fever. Elevated temperature following misoprostol administration is a concerning side effect that may require further investigation to rule out an infection, especially if the elevated temperature persists for > 4 hours. The preoptic area of the anterior hypothalamus (POAH) plays a major role in thermoregulation. When an infection causes an increase in endogenous pyrogens, including interleukin-1β, interleukin-6 and tumor necrosis factor, prostaglandins are generated in the region of the POAH, increasing the thermoregulatory set point, triggering cutaneous vasoconstriction and shivering and non-shivering thermogenesis.1 Misoprostol, especially at doses >400 µg commonly causes both patient-reported chills and temperature elevation >38° C.
In a study comparing misoprostol and oxytocin for the management of the third stage of labor, 597 patients were randomly allocated to receive oxytocin 10 units by intramuscular injection or misoprostol 400 µg or 600 µg by the oral route.2 Patient-reported shivering occurred in 13%, 19%, and 28% of patients receiving oxytocin, misoprostol 400 µg and misoprostol 800 µg, respectively. A recorded temperature >38° C occurred within 1 hour of medication administration in approximately 3%, 2%, and 7.5% of patients receiving oxytocin, misoprostol 400 µg, and misoprostol 800 µg, respectively. In another study, 453 patients scheduled for a cesarean birth were randomly allocated to receive 1 of 3 doses of rectal misoprostol 200 μg, 400 μg, or 600 μg before incision. Fever was detected in 2.6%, 9.9%, and 5.1% of the patients receiving misoprostol 200 μg, 400 μg, or 600 μg, respectively.3
References
1. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304-315. doi: 10.1016/s0002-9343(01)00834-8.
2. Lumbiganon P, Hofmeyr J, Gumezoglu AM, et al. Misoprostol dose-related shivering and pyrexia in the third stage of labor. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labor. Br J Obstet Gynaecol. 1999;106:304-308. doi: 10.1111/j.1471-0528.1999.tb08266.x.
3. Sweed M, El-Said M, Abou-Gamrah AA, et al. Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarean section: a randomized clinical trial. J Obstet Gynaecol Res. 2019;45:585-591. doi: 10.1111 /jog.13883.
- Cytotec [package insert]. Chicago, IL: GD Searle & Co. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf. Accessed June 20, 2022.
- Cervidil [package insert]. St Louis, MO: Forrest Pharmaceuticals Inc.; May 2006. Accessed June 20, 2022.
- Caverject [package insert]. New York, NY: Pfizer Inc.; March 2014. Accessed June 20, 2022.
- Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev. 1990;10:149-172. doi: 10.1002/med.2610100202.
- Audit M, White KI, Breton B, et al. Crystal structure of misoprostol bound to the labor inducer prostaglandin E2 receptor. Nat Chem Biol. 2019;15:11-17. doi: 10.1038/s41589-018-0160-y.
- Pallliser KH, Hirst JJ, Ooi G, et al. Prostaglandin E and F receptor expression and myometrial sensitivity in labor onset in the sheep. Biol Reprod. 2005;72:937-943. doi: 10.1095/biolreprod.104.035311.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280. doi: 10.1016/s0029-7844(98)00436-0.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92. doi: 10.1016/S0029-7844(97)00111-7.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84. doi: 10.1093/humrep/deh005.
- Young DC, Delaney T, Armson BA, et al. Oral misoprostol, low dose vaginal misoprostol and vaginal dinoprostone for labor induction: randomized controlled trial. PLOS One. 2020;15:e0227245. doi: 10.1371/journal.pone.0227245.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01.AOG.0000230398.32794.9d.
- el-Sahwi S, Gaafar AA, Toppozada HK. A new unit for evaluation of uterine activity. Am J Obstet Gynecol. 1967;98:900-903. doi: 10.1016/0002-9378(67)90074-9.
- Vukas N, Ekerhovd E, Abrahamsson G, et al. Cervical priming in the first trimester: morphological and biochemical effects of misoprostol and isosorbide mononitrate. Acta Obstet Gyecol. 2009;88:43-51. doi: 10.1080/00016340802585440.
- Aronsson A, Ulfgren AK, Stabi B, et al. The effect of orally and vaginally administered misoprostol on inflammatory mediators and cervical ripening during early pregnancy. Contraception. 2005;72:33-39. doi: 10.1016/j.contraception.2005.02.012.
- Denison FC, Riley SC, Elliott CL, et al. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6:541-548. doi: 10.1093/molehr/6.6.541.
- ten Eikelder MLG, Rengerink KO, Jozwiak M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet. 2016;387:1619-1628. doi: 10.1016 /S0140-6736(16)00084-2.
- Cytotec [package insert]. Chicago, IL: GD Searle & Co. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf. Accessed June 20, 2022.
- Cervidil [package insert]. St Louis, MO: Forrest Pharmaceuticals Inc.; May 2006. Accessed June 20, 2022.
- Caverject [package insert]. New York, NY: Pfizer Inc.; March 2014. Accessed June 20, 2022.
- Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev. 1990;10:149-172. doi: 10.1002/med.2610100202.
- Audit M, White KI, Breton B, et al. Crystal structure of misoprostol bound to the labor inducer prostaglandin E2 receptor. Nat Chem Biol. 2019;15:11-17. doi: 10.1038/s41589-018-0160-y.
- Pallliser KH, Hirst JJ, Ooi G, et al. Prostaglandin E and F receptor expression and myometrial sensitivity in labor onset in the sheep. Biol Reprod. 2005;72:937-943. doi: 10.1095/biolreprod.104.035311.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280. doi: 10.1016/s0029-7844(98)00436-0.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92. doi: 10.1016/S0029-7844(97)00111-7.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84. doi: 10.1093/humrep/deh005.
- Young DC, Delaney T, Armson BA, et al. Oral misoprostol, low dose vaginal misoprostol and vaginal dinoprostone for labor induction: randomized controlled trial. PLOS One. 2020;15:e0227245. doi: 10.1371/journal.pone.0227245.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01.AOG.0000230398.32794.9d.
- el-Sahwi S, Gaafar AA, Toppozada HK. A new unit for evaluation of uterine activity. Am J Obstet Gynecol. 1967;98:900-903. doi: 10.1016/0002-9378(67)90074-9.
- Vukas N, Ekerhovd E, Abrahamsson G, et al. Cervical priming in the first trimester: morphological and biochemical effects of misoprostol and isosorbide mononitrate. Acta Obstet Gyecol. 2009;88:43-51. doi: 10.1080/00016340802585440.
- Aronsson A, Ulfgren AK, Stabi B, et al. The effect of orally and vaginally administered misoprostol on inflammatory mediators and cervical ripening during early pregnancy. Contraception. 2005;72:33-39. doi: 10.1016/j.contraception.2005.02.012.
- Denison FC, Riley SC, Elliott CL, et al. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6:541-548. doi: 10.1093/molehr/6.6.541.
- ten Eikelder MLG, Rengerink KO, Jozwiak M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet. 2016;387:1619-1628. doi: 10.1016 /S0140-6736(16)00084-2.
