User login
CASE Patient wants minimally invasive surgery for her fibroids, and no hysterectomy
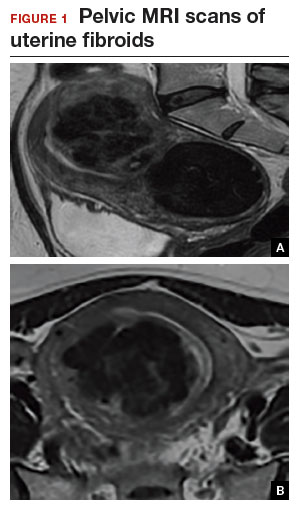
A 44-year-old G1P1 woman comes to the office to discuss her uterine fibroids, heavy menstrual bleeding, and urinary frequency. Treatment with oral contraceptives has not been effective in reducing the bleeding. She now wants surgical treatment without a hysterectomy (the hysterectomy was recommended by her previous gynecologist). On examination, a 14-week-size irregular uterus is felt. Myomectomy is discussed, and the patient asks if minimally invasive surgery (MIS) is possible. Complete blood cell count testing shows a hemoglobin level of 9.4 g/dL. Pelvic magnetic resonance imaging (MRI) shows a 6-cm type 2 posterior fundal fibroid and a 6-cm type 5 posterior lower-uterine-segment fibroid (FIGURE 1). These 2 fibroids have regular contours, and enhancement is not increased with contrast, consistent with benign fibroids.
Determining that laparoscopic myomectomy is a good option
Fibroids may affect quality of life—they may cause heavy menstrual bleeding, pelvic pain or pressure, or urinary frequency or incontinence. For many women who want large or numerous fibroids removed but the uterus preserved, abdominal myomectomy is required. Smaller and less numerous fibroids usually can be managed laparoscopically or with robotic assistance.
A systematic review of 6 randomized, controlled trials comparing laparoscopic and open myomectomy in 576 patients found that, although laparoscopic myomectomy was associated with longer operative time (approximately 13 minutes), it was also linked to less operative blood loss, fewer overall complications, reduced postoperative pain, and faster recovery.1 However, wide application of the laparoscopic approach may be limited by the size and number of fibroids that can be reasonably removed and by the surgical skill needed for fibroid excision and laparoscopic suturing.
Four imaging modalities can be used for fibroids: transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI. TVS is the most readily available and least costly modality used to differentiate fibroids from other pelvic pathology; SIS provides contrast for the endometrial cavity and better defines submucous fibroids; and hysteroscopy detects visually apparent distortion of the cavity. MRI, however, provides the most complete evaluation of size, position, and number of fibroids.
A study comparing TVS, SIS, hysteroscopy, and MRI found that number and position of fibroids were best identified with MRI.2 In addition, with MRI, the proximity of the fibroids and uterus to the bladder, rectum, and iliac bones can be evaluated. As tactility in laparoscopic and robot-assisted surgery is very limited, surgeons who use MRI to accurately assess fibroids preoperatively may be able to avoid missing them during the procedure.3 MRI also can be used reliably to diagnose adenomyosis and may be able to help identify uterine sarcoma.
Tip. For all women considering laparoscopic or robot-assisted myomectomy, I order pelvic MRI with and without contrast. Having the radiologist limit the number of MRI sequences may reduce the cost and make it comparable to that of other imaging modalities. I request T2-weighted MRI scans in the coronal, sagittal, and axial planes; in addition, to determine distortion of the uterine cavity by submucous fibroids, I request scans in the planes parallel with and perpendicular to the uterine axis. One gadolinium-enhanced T1-weighted MRI scan is needed to evaluate perfusion.
Although radiologists are experts in image interpretation, they are unfamiliar with the treatments and surgical issues that gynecologists must consider. Reading MRI scans for fibroids is straightforward, and gynecologists who regularly treat women with fibroids should consider viewing images with a radiologist until they become proficient.
Related article:
Surgical management of broad ligament fibroids
Surgeons who have the experience and skill and know the size, number, and position of fibroids are able to select the appropriate candidates for laparoscopic myomectomy. Authors of a study of 2,050 laparoscopic myomectomies found that fibroids larger than 5 cm, removal of more than 3 fibroids, and broad ligament fibroids were more likely to be associated with major complications, including visceral injury, conversion to laparotomy, and bleeding requiring blood transfusion.4
In laparoscopic myomectomy, uterus reconstruction requires laparoscopic suturing. Although robot-assisted myomectomy may make laparoscopic suturing easier, the added cost, longer operative time, and unimproved outcomes must be considered too.
Read about trocar placement and managing blood loss
Trocar placement
Place the patient in the dorsal lithotomy position.
Tip. For most women, I do not use a uterine manipulator, as my assistant can manipulate the uterus with laparoscopic graspers.
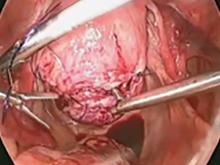
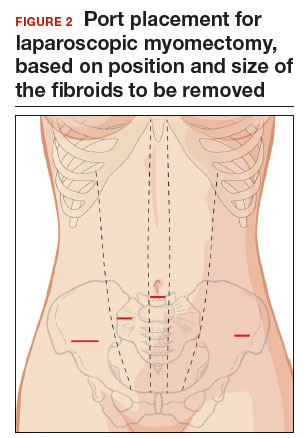
Port placement should be based on the position and size of the fibroids to be removed. Laparoscopic suturing is more ergonomic with 2 ports placed on one side of the patient (FIGURE 2). For suture access, a 12-mm port is placed about 2 cm medial to the iliac crest and a 5-mm port is placed medial to the 12-mm port, near the level of the umbilicus. Lateral trocars should be placed high, above the superior aspect of the uterus, to make it easier to access the fibroids, and lateral to the inferior epigastric vessels, to avoid injuring those vessels. If the uterus is near or above the umbilicus, a left upper quadrant approach may be used, with the access ports placed above the umbilicus.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Managing intraoperative blood loss
I use a combination of 3 agents to reduce intraoperative blood loss during laparoscopic myomectomy: preoperative misoprostol and tranexamic acid and intraoperative vasopressin. Although there are no data showing an advantage in using these drugs together, the agents have different mechanisms of action and no negative interactions.
Injected below the vascular pseudocapsule, 20 units of vasopressin in 100 mL of normal saline causes vasoconstriction of capillaries, small arterioles, and venules. Avoid intravascular injection given that bradycardia and cardiovascular collapse have been reported (rare cases). Loss of peripheral pulses, bradycardia, unmeasurable blood pressure, and cardiac complications have been reported after myometrial injection of ≥5 units of vasopressin.5
Although vasopressin is a powerful vasoconstrictor, these clinical findings are often interpreted as severe hypotension. However, evaluation of peripheral arterial blood flow by Doppler ultrasonography has revealed severe vasospasm and increased proximal blood pressure.5 Keep this potential reaction in mind to avoid misinterpreting findings and treating a patient with vasopressors. Presence of palpable carotid pulses and maintenance of normal partial pressure of end-tidal carbon dioxide can help differentiate peripheral vasospasm from global hypotension.
Use of vasopressin to reduce blood loss during myomectomy is off-label. On occasion, I apply a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. I use a red Robinson catheter, throw 1 tie in front of the uterus, pull with graspers on both ends until it is tight, and then clamp the half-knot with a locking grasper.
Tip. Although a salvage-type autologous blood transfusion device may be used during laparoscopic or robot-assisted myomectomy, cases in which this device is considered for very large or multiple fibroids might be better managed with abdominal myomectomy.
Surgical technique
After injecting vasopressin, I use a high-frequency mechanical vibration scalpel to incise the myometrium directly over a prominent fibroid and carry the incision deeply until fibroid tissue is definite. Alternatively, a monopolar laparoscopic needle can be used in cut mode—which also limits damage to the myometrium.
Tip. The course of vessels over a fibroid is unpredictable, and we cannot be certain that any uterine incision will avoid bleeding. Therefore, I make transverse incisions, which allow more ergonomic laparoscopic suturing.
It is important to incise completely through the myometrium and through the pink-red pseudocapsule containing the vascular network surrounding the fibroid. This plane is often deeper than usually recognized and can be identified just over the white fibroid.
The fibroid is grasped with a tenaculum for traction, and countertraction is applied with a grasper on the myometrial edges. Once the fibroid is reached, graspers and the mechanical vibration scalpel are used to tease the pseudocapsule away from the fibroid (VIDEO).
Tip. Staying under the pseudocapsule reduces bleeding and may preserve the tissue’s growth factors and neurotransmitters, which are thought to promote wound healing.6
Dissection with the mechanical vibration scalpel (or monopolar needle) should be performed under visual control to identify the tissue adhering to the fibroid, which is desiccated and then divided. The fibroid is dissected until free of the myometrium and is placed in the right lower abdomen. Small fibroids can be strung together on a long suture so none will be lost. Using bipolar paddles, desiccate large bleeding vessels in the myometrial defect sparingly, with care taken to avoid devascularizing the myometrium, which might compromise wound healing. Myometrial repair should be performed in accordance with the accepted surgical technique used in laparotomy.
Place delayed absorbable sutures in 2 or 3 layers, as needed, to reapproximate the myometrium and secure hemostasis.
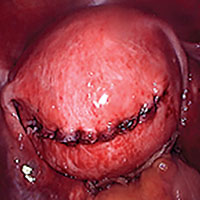
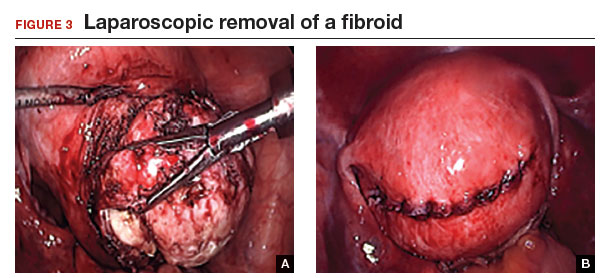
Tip: I use 0 polydioxanone interrupted figure-of-8 sutures, but continuous running sutures with or without barbs also can be used. For the serosa, I use a continuous barbed suture in a baseball stitch, which buries both the raw edges of the serosa and the barbs for smooth closure (FIGURE 3). These closure methods have not been compared to see which provides superior wound healing or subsequent wound strength.
The fibroid can be morcellated with an electromechanical morcellator or a scalpel (hand morcellation). Either instrument can be used in contained or uncontained fashion. I insert an electromechanical morcellator through the right lower quadrant incision and morcellate tissue in the anterior midpelvis. Safety requires careful control of the rotating blade and scrutiny of the bowel, bladder, and major vessels. Our operating room has 4 rules for morcellator use:
- The blade is activated only under direct visualization.
- Both the surgeon and the assistant must say “ready” before the blade is activated.
- The hand holding the morcellator must remain still while tissue is being drawn into the device.
- Any undue resistance from the tissue is cause to stop the blade. This precaution is taken because there is a tendency to drop the blade in an attempt to overcome the resistance.
Tip: I limit rotational forces and scattering of tissue by “pulsing” the blade on and off when morcellating softer tissue.
Various methods of contained morcellation (morcellation in a containment bag) have been described.7 In one method, tissue is placed in a bag, the neck of the bag is brought through an enlarged umbilical incision, and the tissue is cut into small pieces until it is entirely removed. Another method is to use an electromechanical morcellator with a specially designed containment bag inside the abdomen. The bag is introduced through a 12-mm port and unfurled inside the abdomen; the specimen is placed in the bag; the neck of the bag is brought out through the port; the bag is insufflated with carbon dioxide; the laparoscope, a 5-mm grasper, and the morcellator tip are passed into the bag; and morcellation is performed. Early studies of contained morcellation reported longer operating times, leaking bags, and visceral injuries. In 2016, the US Food and Drug Administration (FDA) cleared the PneumoLiner containment system but required that its manufacturer (Advanced Surgical Concepts) warn patients and health care providers that its bag has not been proved to reduce the risk of spreading cancer during morcellation procedures.8
During laparoscopic myomectomy, fibroid removal by myometrial dissection disperses tissue fragments, and the unprotected fibroid is usually stored in the abdomen until hemostasis is secured and suturing completed. Limiting the rotational forces that lead to further dispersement and irrigating copiously to remove tissue fragments help eliminate residual tissue.
The pelvis and the abdomen are irrigated with normal saline (approximately 3 L) and suctioned multiple times.
Tip. Alternating between the Trendelenburg and reverse Trendelenburg positions allows fluid to wash tissue down to the pelvis, where it is more easily seen and removed.
Careful inspection for tissue fragments and copious irrigation and suctioning are important in reducing the risk that tissue fragments will remain in the peritoneal cavity and parasitic fibroids will develop. In cases of occult leiomyosarcoma (LMS), this step may be particularly important.
I place a knitted fabric of modified cellulose over the hysterotomy suture lines to reduce the incidence of adhesion formation. Once the procedure is complete, the local anesthetic bupivicaine is injected deep into the incision sites. Injecting anesthetic before making the incisions does not provide better pain relief; injecting after the procedure provides pain relief for 6 hours.9
Related article:
Robot-assisted laparoscopic myomectomy
Morcellation and risk of leiomyosarcoma
Given the need to prevent laparoscopic morcellators from inadvertently spreading tissue within the peritoneal cavity of women with occult LMS, the FDA issued a safety communication in 2014 warning against their use in the majority of women who undergo myomectomy or hysterectomy for fibroids.10 However, Pritts and colleagues estimated the prevalence of LMS in women who had surgery for presumed uterine fibroids at about 1 in 2,000 (0.05%), significantly lower than the FDA’s estimate of 1 in 350.10,11 In 2015, a large population-based prospective registry study found 2 cases of occult LMS in 8,720 fibroid surgery patients (0.02%).12
Related article:
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Since LMS metastasizes through the bloodstream, there is no reliable evidence that morcellation influences survival or that electromechanical morcellation is inferior to vaginal or mini-laparotomy morcellation with a scalpel. According to recent publications, compared with MIS, open abdominal surgery is associated with more morbidity and mortality in women.13 Since the FDA advisory was issued, the number of abdominal surgeries has increased, as has the number of related complications.13
I use electromechanical morcellation techniques for women who want MIS. All surgical procedures have potential risks, and patients’ and physicians’ understanding of risks forms the foundation of medical decision making. The possibility of occult LMS should be considered by women and their gynecologists, and proper informed consent, noting both the LMS risk and the increased risks of abdominal surgery, should be obtained.
Related article:
Tissue extraction: Can the pendulum change direction?
Risk of uterine rupture after laparoscopic myomectomy
After abdominal myomectomy, uterine rupture during pregnancy or delivery is rare, according to reviews of delivery records of many thousands of women.14 Operative techniques, instruments, and energy sources used during laparoscopic or robot-assisted myomectomy may differ from those used during laparotomy, and anecdotal communications suggest that uterine rupture may be more common after laparoscopic or robot-assisted myomectomy. A meta-analysis of 56 articles (3,685 pregnancies) published between 1970 and 2013 found 29 cases of uterine rupture after myomectomy, with no statistical difference in rupture risk between laparoscopic and abdominal myomectomy.15 As most reports are case studies or small case series, the incidence of rupture cannot be reliably calculated.
There is no consensus regarding the factors that may increase the risk of uterine rupture after laparoscopic myomectomy. Three factors are postulated to interfere with myometrial wound healing and increase uterine rupture risk: failure to adequately suture myometrial defects, excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium, and lack of hemostasis with subsequent hematoma formation.16 It seems prudent that surgeons should adhere to time-tested techniques for abdominal myomectomy. Even with use of ideal surgical techniques, however, individual wound-healing characteristics may predispose to uterine rupture.
CASE Resolved
After giving proper informed consent, the patient underwent laparoscopic myomectomy and electromechanical morcellation. Her 2 fibroids were removed, with a blood loss of 200 mL, and that afternoon she was discharged from the surgery center with written postoperative instructions and oral pain medication. A telephone call the next day found her comfortable, with no nausea or vomiting, and happy to be fibroid free. Pathologic inspection of the morcellated tissue confirmed that the fibroids were benign. At 2-week follow-up, the patient was no longer taking pain medication and was ready to return to work and normal activity. Her fatigue persisted, though, and she arranged to take time to rest during the day.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Parker WH. The utility of MRI for the surgical treatment of women with uterine fibroid tumors. Am J Obstet Gynecol. 2012;206(1):31–36.
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453–462.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2016;18(2):129–139.
- Taylan E, Sahin C, Zeybek B, Akdemir A. Contained morcellation: review of current methods and future directions. Front Surg. 2017;4:15.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Published April 7, 2016. Accessed June 9, 2017.
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD007049.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed June 9, 2017.
- Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. 2015;292(3):665–672.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1–e13.
- Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94(4):571–576.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
CASE Patient wants minimally invasive surgery for her fibroids, and no hysterectomy
A 44-year-old G1P1 woman comes to the office to discuss her uterine fibroids, heavy menstrual bleeding, and urinary frequency. Treatment with oral contraceptives has not been effective in reducing the bleeding. She now wants surgical treatment without a hysterectomy (the hysterectomy was recommended by her previous gynecologist). On examination, a 14-week-size irregular uterus is felt. Myomectomy is discussed, and the patient asks if minimally invasive surgery (MIS) is possible. Complete blood cell count testing shows a hemoglobin level of 9.4 g/dL. Pelvic magnetic resonance imaging (MRI) shows a 6-cm type 2 posterior fundal fibroid and a 6-cm type 5 posterior lower-uterine-segment fibroid (FIGURE 1). These 2 fibroids have regular contours, and enhancement is not increased with contrast, consistent with benign fibroids.

Determining that laparoscopic myomectomy is a good option
Fibroids may affect quality of life—they may cause heavy menstrual bleeding, pelvic pain or pressure, or urinary frequency or incontinence. For many women who want large or numerous fibroids removed but the uterus preserved, abdominal myomectomy is required. Smaller and less numerous fibroids usually can be managed laparoscopically or with robotic assistance.
A systematic review of 6 randomized, controlled trials comparing laparoscopic and open myomectomy in 576 patients found that, although laparoscopic myomectomy was associated with longer operative time (approximately 13 minutes), it was also linked to less operative blood loss, fewer overall complications, reduced postoperative pain, and faster recovery.1 However, wide application of the laparoscopic approach may be limited by the size and number of fibroids that can be reasonably removed and by the surgical skill needed for fibroid excision and laparoscopic suturing.
Four imaging modalities can be used for fibroids: transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI. TVS is the most readily available and least costly modality used to differentiate fibroids from other pelvic pathology; SIS provides contrast for the endometrial cavity and better defines submucous fibroids; and hysteroscopy detects visually apparent distortion of the cavity. MRI, however, provides the most complete evaluation of size, position, and number of fibroids.
A study comparing TVS, SIS, hysteroscopy, and MRI found that number and position of fibroids were best identified with MRI.2 In addition, with MRI, the proximity of the fibroids and uterus to the bladder, rectum, and iliac bones can be evaluated. As tactility in laparoscopic and robot-assisted surgery is very limited, surgeons who use MRI to accurately assess fibroids preoperatively may be able to avoid missing them during the procedure.3 MRI also can be used reliably to diagnose adenomyosis and may be able to help identify uterine sarcoma.
Tip. For all women considering laparoscopic or robot-assisted myomectomy, I order pelvic MRI with and without contrast. Having the radiologist limit the number of MRI sequences may reduce the cost and make it comparable to that of other imaging modalities. I request T2-weighted MRI scans in the coronal, sagittal, and axial planes; in addition, to determine distortion of the uterine cavity by submucous fibroids, I request scans in the planes parallel with and perpendicular to the uterine axis. One gadolinium-enhanced T1-weighted MRI scan is needed to evaluate perfusion.
Although radiologists are experts in image interpretation, they are unfamiliar with the treatments and surgical issues that gynecologists must consider. Reading MRI scans for fibroids is straightforward, and gynecologists who regularly treat women with fibroids should consider viewing images with a radiologist until they become proficient.
Related article:
Surgical management of broad ligament fibroids
Surgeons who have the experience and skill and know the size, number, and position of fibroids are able to select the appropriate candidates for laparoscopic myomectomy. Authors of a study of 2,050 laparoscopic myomectomies found that fibroids larger than 5 cm, removal of more than 3 fibroids, and broad ligament fibroids were more likely to be associated with major complications, including visceral injury, conversion to laparotomy, and bleeding requiring blood transfusion.4
In laparoscopic myomectomy, uterus reconstruction requires laparoscopic suturing. Although robot-assisted myomectomy may make laparoscopic suturing easier, the added cost, longer operative time, and unimproved outcomes must be considered too.

Read about trocar placement and managing blood loss
Trocar placement
Place the patient in the dorsal lithotomy position.
Tip. For most women, I do not use a uterine manipulator, as my assistant can manipulate the uterus with laparoscopic graspers.
Port placement should be based on the position and size of the fibroids to be removed. Laparoscopic suturing is more ergonomic with 2 ports placed on one side of the patient (FIGURE 2). For suture access, a 12-mm port is placed about 2 cm medial to the iliac crest and a 5-mm port is placed medial to the 12-mm port, near the level of the umbilicus. Lateral trocars should be placed high, above the superior aspect of the uterus, to make it easier to access the fibroids, and lateral to the inferior epigastric vessels, to avoid injuring those vessels. If the uterus is near or above the umbilicus, a left upper quadrant approach may be used, with the access ports placed above the umbilicus.

Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Managing intraoperative blood loss
I use a combination of 3 agents to reduce intraoperative blood loss during laparoscopic myomectomy: preoperative misoprostol and tranexamic acid and intraoperative vasopressin. Although there are no data showing an advantage in using these drugs together, the agents have different mechanisms of action and no negative interactions.
Injected below the vascular pseudocapsule, 20 units of vasopressin in 100 mL of normal saline causes vasoconstriction of capillaries, small arterioles, and venules. Avoid intravascular injection given that bradycardia and cardiovascular collapse have been reported (rare cases). Loss of peripheral pulses, bradycardia, unmeasurable blood pressure, and cardiac complications have been reported after myometrial injection of ≥5 units of vasopressin.5
Although vasopressin is a powerful vasoconstrictor, these clinical findings are often interpreted as severe hypotension. However, evaluation of peripheral arterial blood flow by Doppler ultrasonography has revealed severe vasospasm and increased proximal blood pressure.5 Keep this potential reaction in mind to avoid misinterpreting findings and treating a patient with vasopressors. Presence of palpable carotid pulses and maintenance of normal partial pressure of end-tidal carbon dioxide can help differentiate peripheral vasospasm from global hypotension.
Use of vasopressin to reduce blood loss during myomectomy is off-label. On occasion, I apply a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. I use a red Robinson catheter, throw 1 tie in front of the uterus, pull with graspers on both ends until it is tight, and then clamp the half-knot with a locking grasper.
Tip. Although a salvage-type autologous blood transfusion device may be used during laparoscopic or robot-assisted myomectomy, cases in which this device is considered for very large or multiple fibroids might be better managed with abdominal myomectomy.
Surgical technique
After injecting vasopressin, I use a high-frequency mechanical vibration scalpel to incise the myometrium directly over a prominent fibroid and carry the incision deeply until fibroid tissue is definite. Alternatively, a monopolar laparoscopic needle can be used in cut mode—which also limits damage to the myometrium.
Tip. The course of vessels over a fibroid is unpredictable, and we cannot be certain that any uterine incision will avoid bleeding. Therefore, I make transverse incisions, which allow more ergonomic laparoscopic suturing.
It is important to incise completely through the myometrium and through the pink-red pseudocapsule containing the vascular network surrounding the fibroid. This plane is often deeper than usually recognized and can be identified just over the white fibroid.
The fibroid is grasped with a tenaculum for traction, and countertraction is applied with a grasper on the myometrial edges. Once the fibroid is reached, graspers and the mechanical vibration scalpel are used to tease the pseudocapsule away from the fibroid (VIDEO).
Tip. Staying under the pseudocapsule reduces bleeding and may preserve the tissue’s growth factors and neurotransmitters, which are thought to promote wound healing.6
Dissection with the mechanical vibration scalpel (or monopolar needle) should be performed under visual control to identify the tissue adhering to the fibroid, which is desiccated and then divided. The fibroid is dissected until free of the myometrium and is placed in the right lower abdomen. Small fibroids can be strung together on a long suture so none will be lost. Using bipolar paddles, desiccate large bleeding vessels in the myometrial defect sparingly, with care taken to avoid devascularizing the myometrium, which might compromise wound healing. Myometrial repair should be performed in accordance with the accepted surgical technique used in laparotomy.
Place delayed absorbable sutures in 2 or 3 layers, as needed, to reapproximate the myometrium and secure hemostasis.
Tip: I use 0 polydioxanone interrupted figure-of-8 sutures, but continuous running sutures with or without barbs also can be used. For the serosa, I use a continuous barbed suture in a baseball stitch, which buries both the raw edges of the serosa and the barbs for smooth closure (FIGURE 3). These closure methods have not been compared to see which provides superior wound healing or subsequent wound strength.

The fibroid can be morcellated with an electromechanical morcellator or a scalpel (hand morcellation). Either instrument can be used in contained or uncontained fashion. I insert an electromechanical morcellator through the right lower quadrant incision and morcellate tissue in the anterior midpelvis. Safety requires careful control of the rotating blade and scrutiny of the bowel, bladder, and major vessels. Our operating room has 4 rules for morcellator use:
- The blade is activated only under direct visualization.
- Both the surgeon and the assistant must say “ready” before the blade is activated.
- The hand holding the morcellator must remain still while tissue is being drawn into the device.
- Any undue resistance from the tissue is cause to stop the blade. This precaution is taken because there is a tendency to drop the blade in an attempt to overcome the resistance.
Tip: I limit rotational forces and scattering of tissue by “pulsing” the blade on and off when morcellating softer tissue.
Various methods of contained morcellation (morcellation in a containment bag) have been described.7 In one method, tissue is placed in a bag, the neck of the bag is brought through an enlarged umbilical incision, and the tissue is cut into small pieces until it is entirely removed. Another method is to use an electromechanical morcellator with a specially designed containment bag inside the abdomen. The bag is introduced through a 12-mm port and unfurled inside the abdomen; the specimen is placed in the bag; the neck of the bag is brought out through the port; the bag is insufflated with carbon dioxide; the laparoscope, a 5-mm grasper, and the morcellator tip are passed into the bag; and morcellation is performed. Early studies of contained morcellation reported longer operating times, leaking bags, and visceral injuries. In 2016, the US Food and Drug Administration (FDA) cleared the PneumoLiner containment system but required that its manufacturer (Advanced Surgical Concepts) warn patients and health care providers that its bag has not been proved to reduce the risk of spreading cancer during morcellation procedures.8
During laparoscopic myomectomy, fibroid removal by myometrial dissection disperses tissue fragments, and the unprotected fibroid is usually stored in the abdomen until hemostasis is secured and suturing completed. Limiting the rotational forces that lead to further dispersement and irrigating copiously to remove tissue fragments help eliminate residual tissue.
The pelvis and the abdomen are irrigated with normal saline (approximately 3 L) and suctioned multiple times.
Tip. Alternating between the Trendelenburg and reverse Trendelenburg positions allows fluid to wash tissue down to the pelvis, where it is more easily seen and removed.
Careful inspection for tissue fragments and copious irrigation and suctioning are important in reducing the risk that tissue fragments will remain in the peritoneal cavity and parasitic fibroids will develop. In cases of occult leiomyosarcoma (LMS), this step may be particularly important.
I place a knitted fabric of modified cellulose over the hysterotomy suture lines to reduce the incidence of adhesion formation. Once the procedure is complete, the local anesthetic bupivicaine is injected deep into the incision sites. Injecting anesthetic before making the incisions does not provide better pain relief; injecting after the procedure provides pain relief for 6 hours.9
Related article:
Robot-assisted laparoscopic myomectomy
Morcellation and risk of leiomyosarcoma
Given the need to prevent laparoscopic morcellators from inadvertently spreading tissue within the peritoneal cavity of women with occult LMS, the FDA issued a safety communication in 2014 warning against their use in the majority of women who undergo myomectomy or hysterectomy for fibroids.10 However, Pritts and colleagues estimated the prevalence of LMS in women who had surgery for presumed uterine fibroids at about 1 in 2,000 (0.05%), significantly lower than the FDA’s estimate of 1 in 350.10,11 In 2015, a large population-based prospective registry study found 2 cases of occult LMS in 8,720 fibroid surgery patients (0.02%).12
Related article:
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Since LMS metastasizes through the bloodstream, there is no reliable evidence that morcellation influences survival or that electromechanical morcellation is inferior to vaginal or mini-laparotomy morcellation with a scalpel. According to recent publications, compared with MIS, open abdominal surgery is associated with more morbidity and mortality in women.13 Since the FDA advisory was issued, the number of abdominal surgeries has increased, as has the number of related complications.13
I use electromechanical morcellation techniques for women who want MIS. All surgical procedures have potential risks, and patients’ and physicians’ understanding of risks forms the foundation of medical decision making. The possibility of occult LMS should be considered by women and their gynecologists, and proper informed consent, noting both the LMS risk and the increased risks of abdominal surgery, should be obtained.
Related article:
Tissue extraction: Can the pendulum change direction?
Risk of uterine rupture after laparoscopic myomectomy
After abdominal myomectomy, uterine rupture during pregnancy or delivery is rare, according to reviews of delivery records of many thousands of women.14 Operative techniques, instruments, and energy sources used during laparoscopic or robot-assisted myomectomy may differ from those used during laparotomy, and anecdotal communications suggest that uterine rupture may be more common after laparoscopic or robot-assisted myomectomy. A meta-analysis of 56 articles (3,685 pregnancies) published between 1970 and 2013 found 29 cases of uterine rupture after myomectomy, with no statistical difference in rupture risk between laparoscopic and abdominal myomectomy.15 As most reports are case studies or small case series, the incidence of rupture cannot be reliably calculated.
There is no consensus regarding the factors that may increase the risk of uterine rupture after laparoscopic myomectomy. Three factors are postulated to interfere with myometrial wound healing and increase uterine rupture risk: failure to adequately suture myometrial defects, excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium, and lack of hemostasis with subsequent hematoma formation.16 It seems prudent that surgeons should adhere to time-tested techniques for abdominal myomectomy. Even with use of ideal surgical techniques, however, individual wound-healing characteristics may predispose to uterine rupture.
CASE Resolved
After giving proper informed consent, the patient underwent laparoscopic myomectomy and electromechanical morcellation. Her 2 fibroids were removed, with a blood loss of 200 mL, and that afternoon she was discharged from the surgery center with written postoperative instructions and oral pain medication. A telephone call the next day found her comfortable, with no nausea or vomiting, and happy to be fibroid free. Pathologic inspection of the morcellated tissue confirmed that the fibroids were benign. At 2-week follow-up, the patient was no longer taking pain medication and was ready to return to work and normal activity. Her fatigue persisted, though, and she arranged to take time to rest during the day.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE Patient wants minimally invasive surgery for her fibroids, and no hysterectomy
A 44-year-old G1P1 woman comes to the office to discuss her uterine fibroids, heavy menstrual bleeding, and urinary frequency. Treatment with oral contraceptives has not been effective in reducing the bleeding. She now wants surgical treatment without a hysterectomy (the hysterectomy was recommended by her previous gynecologist). On examination, a 14-week-size irregular uterus is felt. Myomectomy is discussed, and the patient asks if minimally invasive surgery (MIS) is possible. Complete blood cell count testing shows a hemoglobin level of 9.4 g/dL. Pelvic magnetic resonance imaging (MRI) shows a 6-cm type 2 posterior fundal fibroid and a 6-cm type 5 posterior lower-uterine-segment fibroid (FIGURE 1). These 2 fibroids have regular contours, and enhancement is not increased with contrast, consistent with benign fibroids.

Determining that laparoscopic myomectomy is a good option
Fibroids may affect quality of life—they may cause heavy menstrual bleeding, pelvic pain or pressure, or urinary frequency or incontinence. For many women who want large or numerous fibroids removed but the uterus preserved, abdominal myomectomy is required. Smaller and less numerous fibroids usually can be managed laparoscopically or with robotic assistance.
A systematic review of 6 randomized, controlled trials comparing laparoscopic and open myomectomy in 576 patients found that, although laparoscopic myomectomy was associated with longer operative time (approximately 13 minutes), it was also linked to less operative blood loss, fewer overall complications, reduced postoperative pain, and faster recovery.1 However, wide application of the laparoscopic approach may be limited by the size and number of fibroids that can be reasonably removed and by the surgical skill needed for fibroid excision and laparoscopic suturing.
Four imaging modalities can be used for fibroids: transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI. TVS is the most readily available and least costly modality used to differentiate fibroids from other pelvic pathology; SIS provides contrast for the endometrial cavity and better defines submucous fibroids; and hysteroscopy detects visually apparent distortion of the cavity. MRI, however, provides the most complete evaluation of size, position, and number of fibroids.
A study comparing TVS, SIS, hysteroscopy, and MRI found that number and position of fibroids were best identified with MRI.2 In addition, with MRI, the proximity of the fibroids and uterus to the bladder, rectum, and iliac bones can be evaluated. As tactility in laparoscopic and robot-assisted surgery is very limited, surgeons who use MRI to accurately assess fibroids preoperatively may be able to avoid missing them during the procedure.3 MRI also can be used reliably to diagnose adenomyosis and may be able to help identify uterine sarcoma.
Tip. For all women considering laparoscopic or robot-assisted myomectomy, I order pelvic MRI with and without contrast. Having the radiologist limit the number of MRI sequences may reduce the cost and make it comparable to that of other imaging modalities. I request T2-weighted MRI scans in the coronal, sagittal, and axial planes; in addition, to determine distortion of the uterine cavity by submucous fibroids, I request scans in the planes parallel with and perpendicular to the uterine axis. One gadolinium-enhanced T1-weighted MRI scan is needed to evaluate perfusion.
Although radiologists are experts in image interpretation, they are unfamiliar with the treatments and surgical issues that gynecologists must consider. Reading MRI scans for fibroids is straightforward, and gynecologists who regularly treat women with fibroids should consider viewing images with a radiologist until they become proficient.
Related article:
Surgical management of broad ligament fibroids
Surgeons who have the experience and skill and know the size, number, and position of fibroids are able to select the appropriate candidates for laparoscopic myomectomy. Authors of a study of 2,050 laparoscopic myomectomies found that fibroids larger than 5 cm, removal of more than 3 fibroids, and broad ligament fibroids were more likely to be associated with major complications, including visceral injury, conversion to laparotomy, and bleeding requiring blood transfusion.4
In laparoscopic myomectomy, uterus reconstruction requires laparoscopic suturing. Although robot-assisted myomectomy may make laparoscopic suturing easier, the added cost, longer operative time, and unimproved outcomes must be considered too.

Read about trocar placement and managing blood loss
Trocar placement
Place the patient in the dorsal lithotomy position.
Tip. For most women, I do not use a uterine manipulator, as my assistant can manipulate the uterus with laparoscopic graspers.
Port placement should be based on the position and size of the fibroids to be removed. Laparoscopic suturing is more ergonomic with 2 ports placed on one side of the patient (FIGURE 2). For suture access, a 12-mm port is placed about 2 cm medial to the iliac crest and a 5-mm port is placed medial to the 12-mm port, near the level of the umbilicus. Lateral trocars should be placed high, above the superior aspect of the uterus, to make it easier to access the fibroids, and lateral to the inferior epigastric vessels, to avoid injuring those vessels. If the uterus is near or above the umbilicus, a left upper quadrant approach may be used, with the access ports placed above the umbilicus.

Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Managing intraoperative blood loss
I use a combination of 3 agents to reduce intraoperative blood loss during laparoscopic myomectomy: preoperative misoprostol and tranexamic acid and intraoperative vasopressin. Although there are no data showing an advantage in using these drugs together, the agents have different mechanisms of action and no negative interactions.
Injected below the vascular pseudocapsule, 20 units of vasopressin in 100 mL of normal saline causes vasoconstriction of capillaries, small arterioles, and venules. Avoid intravascular injection given that bradycardia and cardiovascular collapse have been reported (rare cases). Loss of peripheral pulses, bradycardia, unmeasurable blood pressure, and cardiac complications have been reported after myometrial injection of ≥5 units of vasopressin.5
Although vasopressin is a powerful vasoconstrictor, these clinical findings are often interpreted as severe hypotension. However, evaluation of peripheral arterial blood flow by Doppler ultrasonography has revealed severe vasospasm and increased proximal blood pressure.5 Keep this potential reaction in mind to avoid misinterpreting findings and treating a patient with vasopressors. Presence of palpable carotid pulses and maintenance of normal partial pressure of end-tidal carbon dioxide can help differentiate peripheral vasospasm from global hypotension.
Use of vasopressin to reduce blood loss during myomectomy is off-label. On occasion, I apply a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. I use a red Robinson catheter, throw 1 tie in front of the uterus, pull with graspers on both ends until it is tight, and then clamp the half-knot with a locking grasper.
Tip. Although a salvage-type autologous blood transfusion device may be used during laparoscopic or robot-assisted myomectomy, cases in which this device is considered for very large or multiple fibroids might be better managed with abdominal myomectomy.
Surgical technique
After injecting vasopressin, I use a high-frequency mechanical vibration scalpel to incise the myometrium directly over a prominent fibroid and carry the incision deeply until fibroid tissue is definite. Alternatively, a monopolar laparoscopic needle can be used in cut mode—which also limits damage to the myometrium.
Tip. The course of vessels over a fibroid is unpredictable, and we cannot be certain that any uterine incision will avoid bleeding. Therefore, I make transverse incisions, which allow more ergonomic laparoscopic suturing.
It is important to incise completely through the myometrium and through the pink-red pseudocapsule containing the vascular network surrounding the fibroid. This plane is often deeper than usually recognized and can be identified just over the white fibroid.
The fibroid is grasped with a tenaculum for traction, and countertraction is applied with a grasper on the myometrial edges. Once the fibroid is reached, graspers and the mechanical vibration scalpel are used to tease the pseudocapsule away from the fibroid (VIDEO).
Tip. Staying under the pseudocapsule reduces bleeding and may preserve the tissue’s growth factors and neurotransmitters, which are thought to promote wound healing.6
Dissection with the mechanical vibration scalpel (or monopolar needle) should be performed under visual control to identify the tissue adhering to the fibroid, which is desiccated and then divided. The fibroid is dissected until free of the myometrium and is placed in the right lower abdomen. Small fibroids can be strung together on a long suture so none will be lost. Using bipolar paddles, desiccate large bleeding vessels in the myometrial defect sparingly, with care taken to avoid devascularizing the myometrium, which might compromise wound healing. Myometrial repair should be performed in accordance with the accepted surgical technique used in laparotomy.
Place delayed absorbable sutures in 2 or 3 layers, as needed, to reapproximate the myometrium and secure hemostasis.
Tip: I use 0 polydioxanone interrupted figure-of-8 sutures, but continuous running sutures with or without barbs also can be used. For the serosa, I use a continuous barbed suture in a baseball stitch, which buries both the raw edges of the serosa and the barbs for smooth closure (FIGURE 3). These closure methods have not been compared to see which provides superior wound healing or subsequent wound strength.

The fibroid can be morcellated with an electromechanical morcellator or a scalpel (hand morcellation). Either instrument can be used in contained or uncontained fashion. I insert an electromechanical morcellator through the right lower quadrant incision and morcellate tissue in the anterior midpelvis. Safety requires careful control of the rotating blade and scrutiny of the bowel, bladder, and major vessels. Our operating room has 4 rules for morcellator use:
- The blade is activated only under direct visualization.
- Both the surgeon and the assistant must say “ready” before the blade is activated.
- The hand holding the morcellator must remain still while tissue is being drawn into the device.
- Any undue resistance from the tissue is cause to stop the blade. This precaution is taken because there is a tendency to drop the blade in an attempt to overcome the resistance.
Tip: I limit rotational forces and scattering of tissue by “pulsing” the blade on and off when morcellating softer tissue.
Various methods of contained morcellation (morcellation in a containment bag) have been described.7 In one method, tissue is placed in a bag, the neck of the bag is brought through an enlarged umbilical incision, and the tissue is cut into small pieces until it is entirely removed. Another method is to use an electromechanical morcellator with a specially designed containment bag inside the abdomen. The bag is introduced through a 12-mm port and unfurled inside the abdomen; the specimen is placed in the bag; the neck of the bag is brought out through the port; the bag is insufflated with carbon dioxide; the laparoscope, a 5-mm grasper, and the morcellator tip are passed into the bag; and morcellation is performed. Early studies of contained morcellation reported longer operating times, leaking bags, and visceral injuries. In 2016, the US Food and Drug Administration (FDA) cleared the PneumoLiner containment system but required that its manufacturer (Advanced Surgical Concepts) warn patients and health care providers that its bag has not been proved to reduce the risk of spreading cancer during morcellation procedures.8
During laparoscopic myomectomy, fibroid removal by myometrial dissection disperses tissue fragments, and the unprotected fibroid is usually stored in the abdomen until hemostasis is secured and suturing completed. Limiting the rotational forces that lead to further dispersement and irrigating copiously to remove tissue fragments help eliminate residual tissue.
The pelvis and the abdomen are irrigated with normal saline (approximately 3 L) and suctioned multiple times.
Tip. Alternating between the Trendelenburg and reverse Trendelenburg positions allows fluid to wash tissue down to the pelvis, where it is more easily seen and removed.
Careful inspection for tissue fragments and copious irrigation and suctioning are important in reducing the risk that tissue fragments will remain in the peritoneal cavity and parasitic fibroids will develop. In cases of occult leiomyosarcoma (LMS), this step may be particularly important.
I place a knitted fabric of modified cellulose over the hysterotomy suture lines to reduce the incidence of adhesion formation. Once the procedure is complete, the local anesthetic bupivicaine is injected deep into the incision sites. Injecting anesthetic before making the incisions does not provide better pain relief; injecting after the procedure provides pain relief for 6 hours.9
Related article:
Robot-assisted laparoscopic myomectomy
Morcellation and risk of leiomyosarcoma
Given the need to prevent laparoscopic morcellators from inadvertently spreading tissue within the peritoneal cavity of women with occult LMS, the FDA issued a safety communication in 2014 warning against their use in the majority of women who undergo myomectomy or hysterectomy for fibroids.10 However, Pritts and colleagues estimated the prevalence of LMS in women who had surgery for presumed uterine fibroids at about 1 in 2,000 (0.05%), significantly lower than the FDA’s estimate of 1 in 350.10,11 In 2015, a large population-based prospective registry study found 2 cases of occult LMS in 8,720 fibroid surgery patients (0.02%).12
Related article:
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Since LMS metastasizes through the bloodstream, there is no reliable evidence that morcellation influences survival or that electromechanical morcellation is inferior to vaginal or mini-laparotomy morcellation with a scalpel. According to recent publications, compared with MIS, open abdominal surgery is associated with more morbidity and mortality in women.13 Since the FDA advisory was issued, the number of abdominal surgeries has increased, as has the number of related complications.13
I use electromechanical morcellation techniques for women who want MIS. All surgical procedures have potential risks, and patients’ and physicians’ understanding of risks forms the foundation of medical decision making. The possibility of occult LMS should be considered by women and their gynecologists, and proper informed consent, noting both the LMS risk and the increased risks of abdominal surgery, should be obtained.
Related article:
Tissue extraction: Can the pendulum change direction?
Risk of uterine rupture after laparoscopic myomectomy
After abdominal myomectomy, uterine rupture during pregnancy or delivery is rare, according to reviews of delivery records of many thousands of women.14 Operative techniques, instruments, and energy sources used during laparoscopic or robot-assisted myomectomy may differ from those used during laparotomy, and anecdotal communications suggest that uterine rupture may be more common after laparoscopic or robot-assisted myomectomy. A meta-analysis of 56 articles (3,685 pregnancies) published between 1970 and 2013 found 29 cases of uterine rupture after myomectomy, with no statistical difference in rupture risk between laparoscopic and abdominal myomectomy.15 As most reports are case studies or small case series, the incidence of rupture cannot be reliably calculated.
There is no consensus regarding the factors that may increase the risk of uterine rupture after laparoscopic myomectomy. Three factors are postulated to interfere with myometrial wound healing and increase uterine rupture risk: failure to adequately suture myometrial defects, excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium, and lack of hemostasis with subsequent hematoma formation.16 It seems prudent that surgeons should adhere to time-tested techniques for abdominal myomectomy. Even with use of ideal surgical techniques, however, individual wound-healing characteristics may predispose to uterine rupture.
CASE Resolved
After giving proper informed consent, the patient underwent laparoscopic myomectomy and electromechanical morcellation. Her 2 fibroids were removed, with a blood loss of 200 mL, and that afternoon she was discharged from the surgery center with written postoperative instructions and oral pain medication. A telephone call the next day found her comfortable, with no nausea or vomiting, and happy to be fibroid free. Pathologic inspection of the morcellated tissue confirmed that the fibroids were benign. At 2-week follow-up, the patient was no longer taking pain medication and was ready to return to work and normal activity. Her fatigue persisted, though, and she arranged to take time to rest during the day.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Parker WH. The utility of MRI for the surgical treatment of women with uterine fibroid tumors. Am J Obstet Gynecol. 2012;206(1):31–36.
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453–462.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2016;18(2):129–139.
- Taylan E, Sahin C, Zeybek B, Akdemir A. Contained morcellation: review of current methods and future directions. Front Surg. 2017;4:15.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Published April 7, 2016. Accessed June 9, 2017.
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD007049.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed June 9, 2017.
- Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. 2015;292(3):665–672.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1–e13.
- Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94(4):571–576.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Parker WH. The utility of MRI for the surgical treatment of women with uterine fibroid tumors. Am J Obstet Gynecol. 2012;206(1):31–36.
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453–462.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2016;18(2):129–139.
- Taylan E, Sahin C, Zeybek B, Akdemir A. Contained morcellation: review of current methods and future directions. Front Surg. 2017;4:15.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Published April 7, 2016. Accessed June 9, 2017.
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD007049.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed June 9, 2017.
- Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. 2015;292(3):665–672.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1–e13.
- Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94(4):571–576.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.