User login
Pyoderma gangrenosum (PG) and hidradenitis suppurativa (HS) are rare chronic inflammatory dermatoses of unknown etiologies that often are refractory to conventional treatments. Multiple case reports have described the coexistence of these 2 diseases in the same patient.1-11 The diagnosis of PG commonly followed the diagnosis of HS by 5 months to 30 years in several of these cases, suggesting that HS may have triggered a possible underlying inflammatory process that resulted in subsequent development of PG. When presenting in the setting of preexisting HS, PG lesions have occurred both in the same sites of the body affected by HS as well as in distinct sites such as the legs.1
Although the treatment of either PG or HS alone can be difficult, the combination of these 2 diseases presents a further therapeutic challenge. Tumor necrosis factor a (TNF-α) has been implicated in the pathogenesis of both of these diseases, and several cases have demonstrated great potential for the use of TNF-α inhibitors, particularly infliximab, in the treatment of resistant cases of each of these conditions.2,12 We report a case of severe concurrent PG and HS in a 51-year-old man that was refractory to other treatments but clinically remitted in response to infliximab therapy.
Case Report
A 51-year-old man was transferred to our institution from an outside hospital where he had presented with fevers and a worsening rash that had prevented him from ambulating for several days secondary to severe pain. At the outside facility, he was noted to have extensive ulcerations with granulation tissue on the scalp, face, sacrum, buttocks, and bilateral legs. A skin biopsy performed at the outside facility revealed a neutrophilic dermal infiltrate with abscesses and granulation tissue consistent with PG. He was transferred to our institution for continued local wound care, corticosteroid administration, and hyperbaric oxygen therapy.
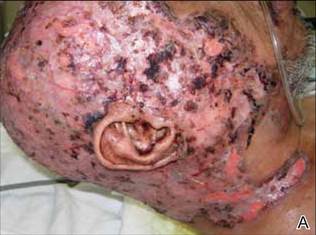
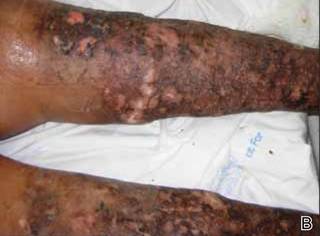
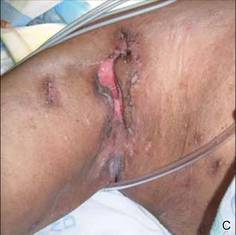
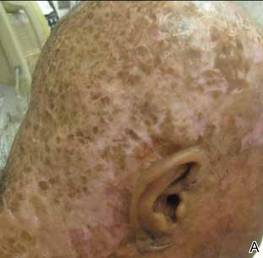
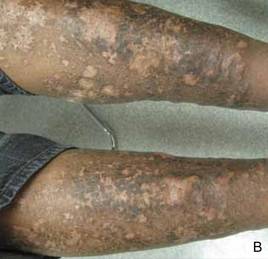
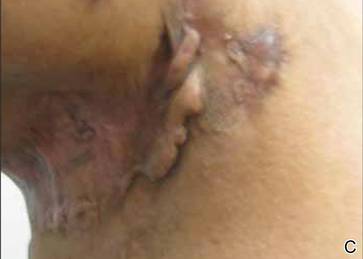
On presentation at our institution, continued ulcerative skin lesions were noted in the previously mentioned areas (Figures 1A and 1B), and deeper purulent sinus tracts were noted in the axillae and extending onto the chest (Figure 1C). A biopsy from the chest showed folliculitis with a prominent plasmacytic infiltrate consistent with HS. Cultures from the sinus tracts showed abundant growth of Enterobacter aerogenes, Klebsiella pneumoniae, Enterobacter cloacae, and Proteus mirabilis. The patient was subsequently treated with intravenous ampicillin-sulbactam and vancomycin as well as oral prednisone 80 mg daily for 2 weeks with only mild improvement of the lesions. The prednisone was gradually tapered to a dose of 10 mg daily in preparation for a trial of infliximab. During the early course of his therapy, subsequent cultures from draining sinus tracts grew Pseudomonas aeruginosa and Acinetobacter baumannii, requiring concomitant treatment with oral antimicrobials including doxycycline, sulfamethoxazole-trimethoprim, ciprofloxacin, and amoxicillin–clavulanic acid for control of the superinfection.
|
The patient received 3 induction infusions of infliximab at a dosage of 5 mg/kg per treatment at weeks 0, 2, and 6. At the time of the first treatment, he was noted to have numerous crusted draining erosions on the head, neck, and chest. The patient reported no adverse effects during or after each treatment. Following completion of the 3 infliximab infusions, the skin lesions showed considerable improvement, with only 1 draining lesion remaining on the left temple and only erythematous plaques remaining on the scalp, upper back, and axillae. Maintenance infusions were continued every 8 weeks with an increased infliximab dose of 7.5 mg/kg.
After 1 year of treatment, the lesions had healed to form cicatrices with no evidence of erythema, drainage, or infection (Figure 2). Doxycycline and prednisone were discontinued, and the infliximab dose was decreased to 5 mg/kg per infusion every 8 weeks. Following sustained improvement after 2 infusions at this lower dose, infliximab was successfully tapered to 2.5 mg/kg every 8 weeks for 2 doses and then was subsequently discontinued; however, the patient’s disease relapsed approximately 7 months after discontinuation of the infliximab and was immediately resumed at a dose of 5 mg/kg per infusion every 8 weeks. He has remained disease free on this dose to date.
|
Comment
Pyoderma gangrenosum is a chronic inflammatory ulcerative skin condition that most commonly occurs on the lower legs.3 In its most common form, PG lesions typically begin as tender erythematous nodules or pustules that evolve into enlarging painful ulcers with raised, undermined, violaceous borders.13 Biopsy specimens taken from the edges of the lesions typically show a diffuse neutrophilic infiltrate, and pseudoepitheliomatous hyperplasia also may be seen. Lesions tend to persist for months to years, ultimately healing as cribriform scars. The etiology of PG is unknown and its pathogenesis is poorly understood.13 Pyoderma gangrenosum is associated with an underlying systemic disease in approximately 50% of cases, most commonly inflammatory bowel disease, hematologic malignancies, and inflammatory arthritis.3 An underlying immunologic abnormality is therefore postulated to contribute to the pathogenesis of PG, as it is frequently associated with these immune-mediated systemic diseases.
There is no specific and uniformly effective treatment of PG, but the main therapeutic goals include the reduction of inflammation to promote wound healing, pain reduction, and the treatment of any comorbid diseases that may contribute to the severity of PG, all while minimizing adverse side effects. Systemic corticosteroids and cyclosporine have been reported to produce the most effective results,13 but due to the side effects and toxicities of these drugs, they are often reserved for severe cases in which the benefits of their use outweigh the risks for other comorbidities. Other reported treatments include antimicrobial agents, topical and intralesional corticosteroids, steroid-sparing immunosuppressive agents, colchicine, hyperbaric oxygen therapy, and more recently drugs that function via immune modulation such as TNF-α inhibitors.
Hidradenitis suppurativa is a chronic suppurative inflammatory disease of follicular occlusion in apocrine gland–bearing areas of the skin such as the groin, axillae, and anogenital region. Hidradenitis suppurativa is characterized by recurrent skin abscesses, sinus tract and fistula formation, and subsequent fibrosis with bacterial overgrowth as a common secondary process.14 The pathogenesis of HS remains poorly understood, but histology typically shows a nonspecific inflammatory process with or without concomitant infection.15 Treatment of HS is complex and usually is transiently effective at best.14 In 2012, Rambhatla et al16 discussed the efficacy of various treatments of HS, including systemic and topical antimicrobial agents (eg, clindamycin-rifampicin combination treatment, tetracycline, topical clindamycin phosphate, isotretinoin, dapsone), antiandrogenic agents, biologic agents (eg, infliximab, etanercept, adalimumab, efalizumab), laser surgery (CO2 laser, Nd:YAG laser), and excisional surgery of sinus tracts. Other therapies include cryotherapy, photodynamic therapy, finasteride, zinc gluconate, topical resorcinol, and acitretin.16
Both PG and HS are categorized as chronic inflammatory disorders with nonspecific histopathologic findings. Although the etiologies of these 2 disorders remain poorly understood, several case reports have suggested an association between PG and HS.1,4 In many of the reported cases of concurrent HS and PG, HS preceded the diagnosis of PG by several years and no correlation in disease activity was observed between the 2 conditions.1-4,6-8 Additionally, the clinical triad of PG, acne, and suppurative hidradenitis, known as PASH syndrome, has been described, which may represent a new entity within the spectrum of autoinflammatory syndromes.5 It is not uncommon for either PG or HS to be refractory to conventional therapies, and therefore the management of concomitant PG and HS presents an even further therapeutic challenge.
Recently, biologic agents such as TNF-α inhibitors have been used with increased frequency as novel treatments for severe dermatologic diseases including psoriasis and pemphigus vulgaris, among others.17 Furthermore, several reports have presented convincing results in the off-label use of TNF-α inhibitors in the treatment of isolated PG as well as in the treatment of HS.12,14,17-22 Infliximab, a chimeric anti–TNF-α monoclonal antibody, has been used with particular success in recalcitrant cases of these conditions.12,17,19-21
In a double-blind, placebo-controlled, crossover trial analyzing 38 patients with moderate to severe refractory HS, Grant et al19 found that treatment with infliximab was associated with a significantly greater improvement in pain intensity (P<.001), disease severity (P<.001), and quality of life (P=.003), with concomitant reduction in clinical markers of inflammation compared to placebo. Similarly, a randomized, double-blind, placebo-controlled trial by Brooklyn et al12 evaluated 30 patients with refractory PG. In this study, infliximab was shown to be superior to placebo for rapid clinical response in patients with PG irrespective of the existence of concomitant inflammatory bowel disease.12
On the contrary, lack of efficacy and/or adverse reactions associated with infliximab and other TNF-α inhibitors in the treatment of PG and HS also have been reported in the literature. Fardet et al20 reported that 2 of 7 (28.6%) HS patients treated with at least 3 infusions of infliximab (5 mg/kg) at weeks 0, 2, and 6 had minimal or nonexistent improvement by week 6. Additionally, adverse events occurred in 3 of 7 (42.9%) HS patients, including abdominal pain caused by colon cancer, multifocal motor neuropathy with conduction block, and a severe allergic reaction.20 Usmani et al21 described 1 HS patient who developed an infliximab-induced lupus reaction, 1 who experienced a hypersensitivity reaction to infliximab, and 2 who had poor response to treatment despite 3 infusions. Kleinpenning et al22 reported a case of PG that failed consecutive trials of etanercept and adalimumab. Wolbing et al23 reported a case of septic shock after treatment of PG with infliximab. Shareef et al24 reported progression of IgA gammopathy to myeloma following infliximab treatment for PG in 1 patient.
Of the reported cases of concurrent HS and PG, 3 were treated with TNF-αinhibitors, both alone and in conjunction with other treatment modalities, with varying results. One patient demonstrated a partial response to treatment with etanercept followed by infliximab, 1 was resistant to treatment with infliximab as well as adalimumab, and 1 exhibited clinical improvement following treatment with infliximab.1,2
Conclusion
In our patient with concurrent PG and HS, both conditions showed dramatic improvement with in-fliximab therapy, and this response has been sustained on 5-mg/kg infliximab maintenance therapy every 8 weeks for the last 3 years. Our case suggests that infliximab may represent an effective therapeutic option for the treatment of concurrent PG and HS that is refractory to conventional therapies. It is yet to be seen whether our patient will continue to experience sustained remission in both his PG and HS permitting the discontinuation of infliximab. Continued study of infliximab and other TNF-α inhibitors is necessary to establish their long-term safety and efficacy for use in patients affected by both HS and PG.
1. Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurative and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265-1270.
2. Moschella S. Is there a role for infliximab in the current therapy of hidradenitis suppurativa? a report of three treated cases. Int J Dermatol. 2007;46:1287-1291.
3. Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
4. Ah-Weng A, Langtry JAA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol. 2005;30:669-671.
5. Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
6. Buckley DA, Rogers S. Cyclosporin-responsive hidradenitis suppurativa. J R Soc Med. 1995;88:289-290.
7. Shenefelt PD. Pyoderma gangrenosum associated with cystic acne and hidradenitis suppurativa controlled by adding minocycline and sulfasalazine to the treatment regimen. Cutis. 1996;57:315-319.
8. Raynor A, Askari AD. Behçet’s disease and treatment with colchicine. J Am Acad Dermatol. 1980;2:396-400.
9. Steinhoff JP, Cilursu A, Falasca GF, et al. A study of musculoskeletal manifestations in 12 patients with SAPHO syndrome. J Clin Rheumatol. 2002;8:13-22.
10. Rosner IA, Richter DE, Huettner TL, et al. Spondyloarthropathy associated with hidradenitis suppurative and acne conglobata. Ann Intern Med. 1982;97:520-525.
11. von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol. 1997;137:1000-1005.
12. Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomized, double-blind, placebo-controlled trial. Gut. 2006;55:505-509.
13. Ruocco E, Sangiulliano S, Gravina AG, et al. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23:1008-1017.
14. Mortimer P. Management of hidradenitis suppurativa. Clin Exp Dermatol. 2002;27:328.
15. Blanco R, Martinez-Taboada VM, Villa I, et al. Long-term successful adalimumab therapy in severe hidradenitis suppurativa. Arch Dermatol. 2009;145:580-584.
16. Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatment for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
17. Alecsandru D, Padilla B, Izquierdo JA, et al. Severe refractory hidradenitis suppurativa in an HIV-positive patient successfully treated with infliximab. Arch Dermatol. 2010;146:1343-1345.
18. Alexis A, Strober B. Off-label dermatologic uses of anti-TNF-a therapies. J Cutan Med Surg. 2006;9:296-301.
19. Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217.
20. Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624-628.
21. Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204-205.
22. Kleinpenning MM, Langewouters AM, Van De Kerkhof PC, et al. Severe pyoderma gangrenosum unresponsive to etanercept and adalimumab. J Dermatolog Treat. 2011;22:261-265.
23. Wolbing F, Fierlbeck G, Hotzenecker W, et al. Septic shock after treatment of pyoderma gangrenosum with infliximab. Acta Dermato-Venereologica. 2009;89:93-94.
24. Shareef MS, Munro LR, Owen RG, et al. Progression of IgA gammopathy to myeloma following infliximab treatment for pyoderma gangrenosum. Clin Exp Dermatol. 2012;37:146-148.
Pyoderma gangrenosum (PG) and hidradenitis suppurativa (HS) are rare chronic inflammatory dermatoses of unknown etiologies that often are refractory to conventional treatments. Multiple case reports have described the coexistence of these 2 diseases in the same patient.1-11 The diagnosis of PG commonly followed the diagnosis of HS by 5 months to 30 years in several of these cases, suggesting that HS may have triggered a possible underlying inflammatory process that resulted in subsequent development of PG. When presenting in the setting of preexisting HS, PG lesions have occurred both in the same sites of the body affected by HS as well as in distinct sites such as the legs.1
Although the treatment of either PG or HS alone can be difficult, the combination of these 2 diseases presents a further therapeutic challenge. Tumor necrosis factor a (TNF-α) has been implicated in the pathogenesis of both of these diseases, and several cases have demonstrated great potential for the use of TNF-α inhibitors, particularly infliximab, in the treatment of resistant cases of each of these conditions.2,12 We report a case of severe concurrent PG and HS in a 51-year-old man that was refractory to other treatments but clinically remitted in response to infliximab therapy.
Case Report
A 51-year-old man was transferred to our institution from an outside hospital where he had presented with fevers and a worsening rash that had prevented him from ambulating for several days secondary to severe pain. At the outside facility, he was noted to have extensive ulcerations with granulation tissue on the scalp, face, sacrum, buttocks, and bilateral legs. A skin biopsy performed at the outside facility revealed a neutrophilic dermal infiltrate with abscesses and granulation tissue consistent with PG. He was transferred to our institution for continued local wound care, corticosteroid administration, and hyperbaric oxygen therapy.
On presentation at our institution, continued ulcerative skin lesions were noted in the previously mentioned areas (Figures 1A and 1B), and deeper purulent sinus tracts were noted in the axillae and extending onto the chest (Figure 1C). A biopsy from the chest showed folliculitis with a prominent plasmacytic infiltrate consistent with HS. Cultures from the sinus tracts showed abundant growth of Enterobacter aerogenes, Klebsiella pneumoniae, Enterobacter cloacae, and Proteus mirabilis. The patient was subsequently treated with intravenous ampicillin-sulbactam and vancomycin as well as oral prednisone 80 mg daily for 2 weeks with only mild improvement of the lesions. The prednisone was gradually tapered to a dose of 10 mg daily in preparation for a trial of infliximab. During the early course of his therapy, subsequent cultures from draining sinus tracts grew Pseudomonas aeruginosa and Acinetobacter baumannii, requiring concomitant treatment with oral antimicrobials including doxycycline, sulfamethoxazole-trimethoprim, ciprofloxacin, and amoxicillin–clavulanic acid for control of the superinfection.



|
The patient received 3 induction infusions of infliximab at a dosage of 5 mg/kg per treatment at weeks 0, 2, and 6. At the time of the first treatment, he was noted to have numerous crusted draining erosions on the head, neck, and chest. The patient reported no adverse effects during or after each treatment. Following completion of the 3 infliximab infusions, the skin lesions showed considerable improvement, with only 1 draining lesion remaining on the left temple and only erythematous plaques remaining on the scalp, upper back, and axillae. Maintenance infusions were continued every 8 weeks with an increased infliximab dose of 7.5 mg/kg.
After 1 year of treatment, the lesions had healed to form cicatrices with no evidence of erythema, drainage, or infection (Figure 2). Doxycycline and prednisone were discontinued, and the infliximab dose was decreased to 5 mg/kg per infusion every 8 weeks. Following sustained improvement after 2 infusions at this lower dose, infliximab was successfully tapered to 2.5 mg/kg every 8 weeks for 2 doses and then was subsequently discontinued; however, the patient’s disease relapsed approximately 7 months after discontinuation of the infliximab and was immediately resumed at a dose of 5 mg/kg per infusion every 8 weeks. He has remained disease free on this dose to date.



|
Comment
Pyoderma gangrenosum is a chronic inflammatory ulcerative skin condition that most commonly occurs on the lower legs.3 In its most common form, PG lesions typically begin as tender erythematous nodules or pustules that evolve into enlarging painful ulcers with raised, undermined, violaceous borders.13 Biopsy specimens taken from the edges of the lesions typically show a diffuse neutrophilic infiltrate, and pseudoepitheliomatous hyperplasia also may be seen. Lesions tend to persist for months to years, ultimately healing as cribriform scars. The etiology of PG is unknown and its pathogenesis is poorly understood.13 Pyoderma gangrenosum is associated with an underlying systemic disease in approximately 50% of cases, most commonly inflammatory bowel disease, hematologic malignancies, and inflammatory arthritis.3 An underlying immunologic abnormality is therefore postulated to contribute to the pathogenesis of PG, as it is frequently associated with these immune-mediated systemic diseases.
There is no specific and uniformly effective treatment of PG, but the main therapeutic goals include the reduction of inflammation to promote wound healing, pain reduction, and the treatment of any comorbid diseases that may contribute to the severity of PG, all while minimizing adverse side effects. Systemic corticosteroids and cyclosporine have been reported to produce the most effective results,13 but due to the side effects and toxicities of these drugs, they are often reserved for severe cases in which the benefits of their use outweigh the risks for other comorbidities. Other reported treatments include antimicrobial agents, topical and intralesional corticosteroids, steroid-sparing immunosuppressive agents, colchicine, hyperbaric oxygen therapy, and more recently drugs that function via immune modulation such as TNF-α inhibitors.
Hidradenitis suppurativa is a chronic suppurative inflammatory disease of follicular occlusion in apocrine gland–bearing areas of the skin such as the groin, axillae, and anogenital region. Hidradenitis suppurativa is characterized by recurrent skin abscesses, sinus tract and fistula formation, and subsequent fibrosis with bacterial overgrowth as a common secondary process.14 The pathogenesis of HS remains poorly understood, but histology typically shows a nonspecific inflammatory process with or without concomitant infection.15 Treatment of HS is complex and usually is transiently effective at best.14 In 2012, Rambhatla et al16 discussed the efficacy of various treatments of HS, including systemic and topical antimicrobial agents (eg, clindamycin-rifampicin combination treatment, tetracycline, topical clindamycin phosphate, isotretinoin, dapsone), antiandrogenic agents, biologic agents (eg, infliximab, etanercept, adalimumab, efalizumab), laser surgery (CO2 laser, Nd:YAG laser), and excisional surgery of sinus tracts. Other therapies include cryotherapy, photodynamic therapy, finasteride, zinc gluconate, topical resorcinol, and acitretin.16
Both PG and HS are categorized as chronic inflammatory disorders with nonspecific histopathologic findings. Although the etiologies of these 2 disorders remain poorly understood, several case reports have suggested an association between PG and HS.1,4 In many of the reported cases of concurrent HS and PG, HS preceded the diagnosis of PG by several years and no correlation in disease activity was observed between the 2 conditions.1-4,6-8 Additionally, the clinical triad of PG, acne, and suppurative hidradenitis, known as PASH syndrome, has been described, which may represent a new entity within the spectrum of autoinflammatory syndromes.5 It is not uncommon for either PG or HS to be refractory to conventional therapies, and therefore the management of concomitant PG and HS presents an even further therapeutic challenge.
Recently, biologic agents such as TNF-α inhibitors have been used with increased frequency as novel treatments for severe dermatologic diseases including psoriasis and pemphigus vulgaris, among others.17 Furthermore, several reports have presented convincing results in the off-label use of TNF-α inhibitors in the treatment of isolated PG as well as in the treatment of HS.12,14,17-22 Infliximab, a chimeric anti–TNF-α monoclonal antibody, has been used with particular success in recalcitrant cases of these conditions.12,17,19-21
In a double-blind, placebo-controlled, crossover trial analyzing 38 patients with moderate to severe refractory HS, Grant et al19 found that treatment with infliximab was associated with a significantly greater improvement in pain intensity (P<.001), disease severity (P<.001), and quality of life (P=.003), with concomitant reduction in clinical markers of inflammation compared to placebo. Similarly, a randomized, double-blind, placebo-controlled trial by Brooklyn et al12 evaluated 30 patients with refractory PG. In this study, infliximab was shown to be superior to placebo for rapid clinical response in patients with PG irrespective of the existence of concomitant inflammatory bowel disease.12
On the contrary, lack of efficacy and/or adverse reactions associated with infliximab and other TNF-α inhibitors in the treatment of PG and HS also have been reported in the literature. Fardet et al20 reported that 2 of 7 (28.6%) HS patients treated with at least 3 infusions of infliximab (5 mg/kg) at weeks 0, 2, and 6 had minimal or nonexistent improvement by week 6. Additionally, adverse events occurred in 3 of 7 (42.9%) HS patients, including abdominal pain caused by colon cancer, multifocal motor neuropathy with conduction block, and a severe allergic reaction.20 Usmani et al21 described 1 HS patient who developed an infliximab-induced lupus reaction, 1 who experienced a hypersensitivity reaction to infliximab, and 2 who had poor response to treatment despite 3 infusions. Kleinpenning et al22 reported a case of PG that failed consecutive trials of etanercept and adalimumab. Wolbing et al23 reported a case of septic shock after treatment of PG with infliximab. Shareef et al24 reported progression of IgA gammopathy to myeloma following infliximab treatment for PG in 1 patient.
Of the reported cases of concurrent HS and PG, 3 were treated with TNF-αinhibitors, both alone and in conjunction with other treatment modalities, with varying results. One patient demonstrated a partial response to treatment with etanercept followed by infliximab, 1 was resistant to treatment with infliximab as well as adalimumab, and 1 exhibited clinical improvement following treatment with infliximab.1,2
Conclusion
In our patient with concurrent PG and HS, both conditions showed dramatic improvement with in-fliximab therapy, and this response has been sustained on 5-mg/kg infliximab maintenance therapy every 8 weeks for the last 3 years. Our case suggests that infliximab may represent an effective therapeutic option for the treatment of concurrent PG and HS that is refractory to conventional therapies. It is yet to be seen whether our patient will continue to experience sustained remission in both his PG and HS permitting the discontinuation of infliximab. Continued study of infliximab and other TNF-α inhibitors is necessary to establish their long-term safety and efficacy for use in patients affected by both HS and PG.
Pyoderma gangrenosum (PG) and hidradenitis suppurativa (HS) are rare chronic inflammatory dermatoses of unknown etiologies that often are refractory to conventional treatments. Multiple case reports have described the coexistence of these 2 diseases in the same patient.1-11 The diagnosis of PG commonly followed the diagnosis of HS by 5 months to 30 years in several of these cases, suggesting that HS may have triggered a possible underlying inflammatory process that resulted in subsequent development of PG. When presenting in the setting of preexisting HS, PG lesions have occurred both in the same sites of the body affected by HS as well as in distinct sites such as the legs.1
Although the treatment of either PG or HS alone can be difficult, the combination of these 2 diseases presents a further therapeutic challenge. Tumor necrosis factor a (TNF-α) has been implicated in the pathogenesis of both of these diseases, and several cases have demonstrated great potential for the use of TNF-α inhibitors, particularly infliximab, in the treatment of resistant cases of each of these conditions.2,12 We report a case of severe concurrent PG and HS in a 51-year-old man that was refractory to other treatments but clinically remitted in response to infliximab therapy.
Case Report
A 51-year-old man was transferred to our institution from an outside hospital where he had presented with fevers and a worsening rash that had prevented him from ambulating for several days secondary to severe pain. At the outside facility, he was noted to have extensive ulcerations with granulation tissue on the scalp, face, sacrum, buttocks, and bilateral legs. A skin biopsy performed at the outside facility revealed a neutrophilic dermal infiltrate with abscesses and granulation tissue consistent with PG. He was transferred to our institution for continued local wound care, corticosteroid administration, and hyperbaric oxygen therapy.
On presentation at our institution, continued ulcerative skin lesions were noted in the previously mentioned areas (Figures 1A and 1B), and deeper purulent sinus tracts were noted in the axillae and extending onto the chest (Figure 1C). A biopsy from the chest showed folliculitis with a prominent plasmacytic infiltrate consistent with HS. Cultures from the sinus tracts showed abundant growth of Enterobacter aerogenes, Klebsiella pneumoniae, Enterobacter cloacae, and Proteus mirabilis. The patient was subsequently treated with intravenous ampicillin-sulbactam and vancomycin as well as oral prednisone 80 mg daily for 2 weeks with only mild improvement of the lesions. The prednisone was gradually tapered to a dose of 10 mg daily in preparation for a trial of infliximab. During the early course of his therapy, subsequent cultures from draining sinus tracts grew Pseudomonas aeruginosa and Acinetobacter baumannii, requiring concomitant treatment with oral antimicrobials including doxycycline, sulfamethoxazole-trimethoprim, ciprofloxacin, and amoxicillin–clavulanic acid for control of the superinfection.



|
The patient received 3 induction infusions of infliximab at a dosage of 5 mg/kg per treatment at weeks 0, 2, and 6. At the time of the first treatment, he was noted to have numerous crusted draining erosions on the head, neck, and chest. The patient reported no adverse effects during or after each treatment. Following completion of the 3 infliximab infusions, the skin lesions showed considerable improvement, with only 1 draining lesion remaining on the left temple and only erythematous plaques remaining on the scalp, upper back, and axillae. Maintenance infusions were continued every 8 weeks with an increased infliximab dose of 7.5 mg/kg.
After 1 year of treatment, the lesions had healed to form cicatrices with no evidence of erythema, drainage, or infection (Figure 2). Doxycycline and prednisone were discontinued, and the infliximab dose was decreased to 5 mg/kg per infusion every 8 weeks. Following sustained improvement after 2 infusions at this lower dose, infliximab was successfully tapered to 2.5 mg/kg every 8 weeks for 2 doses and then was subsequently discontinued; however, the patient’s disease relapsed approximately 7 months after discontinuation of the infliximab and was immediately resumed at a dose of 5 mg/kg per infusion every 8 weeks. He has remained disease free on this dose to date.



|
Comment
Pyoderma gangrenosum is a chronic inflammatory ulcerative skin condition that most commonly occurs on the lower legs.3 In its most common form, PG lesions typically begin as tender erythematous nodules or pustules that evolve into enlarging painful ulcers with raised, undermined, violaceous borders.13 Biopsy specimens taken from the edges of the lesions typically show a diffuse neutrophilic infiltrate, and pseudoepitheliomatous hyperplasia also may be seen. Lesions tend to persist for months to years, ultimately healing as cribriform scars. The etiology of PG is unknown and its pathogenesis is poorly understood.13 Pyoderma gangrenosum is associated with an underlying systemic disease in approximately 50% of cases, most commonly inflammatory bowel disease, hematologic malignancies, and inflammatory arthritis.3 An underlying immunologic abnormality is therefore postulated to contribute to the pathogenesis of PG, as it is frequently associated with these immune-mediated systemic diseases.
There is no specific and uniformly effective treatment of PG, but the main therapeutic goals include the reduction of inflammation to promote wound healing, pain reduction, and the treatment of any comorbid diseases that may contribute to the severity of PG, all while minimizing adverse side effects. Systemic corticosteroids and cyclosporine have been reported to produce the most effective results,13 but due to the side effects and toxicities of these drugs, they are often reserved for severe cases in which the benefits of their use outweigh the risks for other comorbidities. Other reported treatments include antimicrobial agents, topical and intralesional corticosteroids, steroid-sparing immunosuppressive agents, colchicine, hyperbaric oxygen therapy, and more recently drugs that function via immune modulation such as TNF-α inhibitors.
Hidradenitis suppurativa is a chronic suppurative inflammatory disease of follicular occlusion in apocrine gland–bearing areas of the skin such as the groin, axillae, and anogenital region. Hidradenitis suppurativa is characterized by recurrent skin abscesses, sinus tract and fistula formation, and subsequent fibrosis with bacterial overgrowth as a common secondary process.14 The pathogenesis of HS remains poorly understood, but histology typically shows a nonspecific inflammatory process with or without concomitant infection.15 Treatment of HS is complex and usually is transiently effective at best.14 In 2012, Rambhatla et al16 discussed the efficacy of various treatments of HS, including systemic and topical antimicrobial agents (eg, clindamycin-rifampicin combination treatment, tetracycline, topical clindamycin phosphate, isotretinoin, dapsone), antiandrogenic agents, biologic agents (eg, infliximab, etanercept, adalimumab, efalizumab), laser surgery (CO2 laser, Nd:YAG laser), and excisional surgery of sinus tracts. Other therapies include cryotherapy, photodynamic therapy, finasteride, zinc gluconate, topical resorcinol, and acitretin.16
Both PG and HS are categorized as chronic inflammatory disorders with nonspecific histopathologic findings. Although the etiologies of these 2 disorders remain poorly understood, several case reports have suggested an association between PG and HS.1,4 In many of the reported cases of concurrent HS and PG, HS preceded the diagnosis of PG by several years and no correlation in disease activity was observed between the 2 conditions.1-4,6-8 Additionally, the clinical triad of PG, acne, and suppurative hidradenitis, known as PASH syndrome, has been described, which may represent a new entity within the spectrum of autoinflammatory syndromes.5 It is not uncommon for either PG or HS to be refractory to conventional therapies, and therefore the management of concomitant PG and HS presents an even further therapeutic challenge.
Recently, biologic agents such as TNF-α inhibitors have been used with increased frequency as novel treatments for severe dermatologic diseases including psoriasis and pemphigus vulgaris, among others.17 Furthermore, several reports have presented convincing results in the off-label use of TNF-α inhibitors in the treatment of isolated PG as well as in the treatment of HS.12,14,17-22 Infliximab, a chimeric anti–TNF-α monoclonal antibody, has been used with particular success in recalcitrant cases of these conditions.12,17,19-21
In a double-blind, placebo-controlled, crossover trial analyzing 38 patients with moderate to severe refractory HS, Grant et al19 found that treatment with infliximab was associated with a significantly greater improvement in pain intensity (P<.001), disease severity (P<.001), and quality of life (P=.003), with concomitant reduction in clinical markers of inflammation compared to placebo. Similarly, a randomized, double-blind, placebo-controlled trial by Brooklyn et al12 evaluated 30 patients with refractory PG. In this study, infliximab was shown to be superior to placebo for rapid clinical response in patients with PG irrespective of the existence of concomitant inflammatory bowel disease.12
On the contrary, lack of efficacy and/or adverse reactions associated with infliximab and other TNF-α inhibitors in the treatment of PG and HS also have been reported in the literature. Fardet et al20 reported that 2 of 7 (28.6%) HS patients treated with at least 3 infusions of infliximab (5 mg/kg) at weeks 0, 2, and 6 had minimal or nonexistent improvement by week 6. Additionally, adverse events occurred in 3 of 7 (42.9%) HS patients, including abdominal pain caused by colon cancer, multifocal motor neuropathy with conduction block, and a severe allergic reaction.20 Usmani et al21 described 1 HS patient who developed an infliximab-induced lupus reaction, 1 who experienced a hypersensitivity reaction to infliximab, and 2 who had poor response to treatment despite 3 infusions. Kleinpenning et al22 reported a case of PG that failed consecutive trials of etanercept and adalimumab. Wolbing et al23 reported a case of septic shock after treatment of PG with infliximab. Shareef et al24 reported progression of IgA gammopathy to myeloma following infliximab treatment for PG in 1 patient.
Of the reported cases of concurrent HS and PG, 3 were treated with TNF-αinhibitors, both alone and in conjunction with other treatment modalities, with varying results. One patient demonstrated a partial response to treatment with etanercept followed by infliximab, 1 was resistant to treatment with infliximab as well as adalimumab, and 1 exhibited clinical improvement following treatment with infliximab.1,2
Conclusion
In our patient with concurrent PG and HS, both conditions showed dramatic improvement with in-fliximab therapy, and this response has been sustained on 5-mg/kg infliximab maintenance therapy every 8 weeks for the last 3 years. Our case suggests that infliximab may represent an effective therapeutic option for the treatment of concurrent PG and HS that is refractory to conventional therapies. It is yet to be seen whether our patient will continue to experience sustained remission in both his PG and HS permitting the discontinuation of infliximab. Continued study of infliximab and other TNF-α inhibitors is necessary to establish their long-term safety and efficacy for use in patients affected by both HS and PG.
1. Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurative and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265-1270.
2. Moschella S. Is there a role for infliximab in the current therapy of hidradenitis suppurativa? a report of three treated cases. Int J Dermatol. 2007;46:1287-1291.
3. Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
4. Ah-Weng A, Langtry JAA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol. 2005;30:669-671.
5. Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
6. Buckley DA, Rogers S. Cyclosporin-responsive hidradenitis suppurativa. J R Soc Med. 1995;88:289-290.
7. Shenefelt PD. Pyoderma gangrenosum associated with cystic acne and hidradenitis suppurativa controlled by adding minocycline and sulfasalazine to the treatment regimen. Cutis. 1996;57:315-319.
8. Raynor A, Askari AD. Behçet’s disease and treatment with colchicine. J Am Acad Dermatol. 1980;2:396-400.
9. Steinhoff JP, Cilursu A, Falasca GF, et al. A study of musculoskeletal manifestations in 12 patients with SAPHO syndrome. J Clin Rheumatol. 2002;8:13-22.
10. Rosner IA, Richter DE, Huettner TL, et al. Spondyloarthropathy associated with hidradenitis suppurative and acne conglobata. Ann Intern Med. 1982;97:520-525.
11. von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol. 1997;137:1000-1005.
12. Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomized, double-blind, placebo-controlled trial. Gut. 2006;55:505-509.
13. Ruocco E, Sangiulliano S, Gravina AG, et al. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23:1008-1017.
14. Mortimer P. Management of hidradenitis suppurativa. Clin Exp Dermatol. 2002;27:328.
15. Blanco R, Martinez-Taboada VM, Villa I, et al. Long-term successful adalimumab therapy in severe hidradenitis suppurativa. Arch Dermatol. 2009;145:580-584.
16. Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatment for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
17. Alecsandru D, Padilla B, Izquierdo JA, et al. Severe refractory hidradenitis suppurativa in an HIV-positive patient successfully treated with infliximab. Arch Dermatol. 2010;146:1343-1345.
18. Alexis A, Strober B. Off-label dermatologic uses of anti-TNF-a therapies. J Cutan Med Surg. 2006;9:296-301.
19. Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217.
20. Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624-628.
21. Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204-205.
22. Kleinpenning MM, Langewouters AM, Van De Kerkhof PC, et al. Severe pyoderma gangrenosum unresponsive to etanercept and adalimumab. J Dermatolog Treat. 2011;22:261-265.
23. Wolbing F, Fierlbeck G, Hotzenecker W, et al. Septic shock after treatment of pyoderma gangrenosum with infliximab. Acta Dermato-Venereologica. 2009;89:93-94.
24. Shareef MS, Munro LR, Owen RG, et al. Progression of IgA gammopathy to myeloma following infliximab treatment for pyoderma gangrenosum. Clin Exp Dermatol. 2012;37:146-148.
1. Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurative and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265-1270.
2. Moschella S. Is there a role for infliximab in the current therapy of hidradenitis suppurativa? a report of three treated cases. Int J Dermatol. 2007;46:1287-1291.
3. Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
4. Ah-Weng A, Langtry JAA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol. 2005;30:669-671.
5. Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
6. Buckley DA, Rogers S. Cyclosporin-responsive hidradenitis suppurativa. J R Soc Med. 1995;88:289-290.
7. Shenefelt PD. Pyoderma gangrenosum associated with cystic acne and hidradenitis suppurativa controlled by adding minocycline and sulfasalazine to the treatment regimen. Cutis. 1996;57:315-319.
8. Raynor A, Askari AD. Behçet’s disease and treatment with colchicine. J Am Acad Dermatol. 1980;2:396-400.
9. Steinhoff JP, Cilursu A, Falasca GF, et al. A study of musculoskeletal manifestations in 12 patients with SAPHO syndrome. J Clin Rheumatol. 2002;8:13-22.
10. Rosner IA, Richter DE, Huettner TL, et al. Spondyloarthropathy associated with hidradenitis suppurative and acne conglobata. Ann Intern Med. 1982;97:520-525.
11. von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol. 1997;137:1000-1005.
12. Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomized, double-blind, placebo-controlled trial. Gut. 2006;55:505-509.
13. Ruocco E, Sangiulliano S, Gravina AG, et al. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23:1008-1017.
14. Mortimer P. Management of hidradenitis suppurativa. Clin Exp Dermatol. 2002;27:328.
15. Blanco R, Martinez-Taboada VM, Villa I, et al. Long-term successful adalimumab therapy in severe hidradenitis suppurativa. Arch Dermatol. 2009;145:580-584.
16. Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatment for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
17. Alecsandru D, Padilla B, Izquierdo JA, et al. Severe refractory hidradenitis suppurativa in an HIV-positive patient successfully treated with infliximab. Arch Dermatol. 2010;146:1343-1345.
18. Alexis A, Strober B. Off-label dermatologic uses of anti-TNF-a therapies. J Cutan Med Surg. 2006;9:296-301.
19. Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217.
20. Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624-628.
21. Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204-205.
22. Kleinpenning MM, Langewouters AM, Van De Kerkhof PC, et al. Severe pyoderma gangrenosum unresponsive to etanercept and adalimumab. J Dermatolog Treat. 2011;22:261-265.
23. Wolbing F, Fierlbeck G, Hotzenecker W, et al. Septic shock after treatment of pyoderma gangrenosum with infliximab. Acta Dermato-Venereologica. 2009;89:93-94.
24. Shareef MS, Munro LR, Owen RG, et al. Progression of IgA gammopathy to myeloma following infliximab treatment for pyoderma gangrenosum. Clin Exp Dermatol. 2012;37:146-148.
Practice Points
- Pyoderma gangrenosum (PG) and hidradenitis suppurativa (HS) are rare chronic inflammatory dermatoses that may coexist in the same patient.
- Infliximab may represent an effective therapeutic option for the treatment of concurrent PG and HS that is refractory to conventional therapies.