User login
• Classify and treat asthma based on the patient’s worst symptom, whether or not it is the symptom that occurs most frequently. C
• Treat patients with poorly controlled asthma aggressively to gain quick control, then scale back slowly to the fewest medications and lowest doses needed to maintain control. A
• Reserve long-acting beta-agonists for use as an adjunct to inhaled corticosteroids for adults with poor baseline pulmonary function tests. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Angela D, a 34-year-old patient, has asthma with recurrent exacerbations. She uses a low-dose inhaled corticosteroid (ICS) daily and an albuterol inhaler, as needed, for shortness of breath or wheezing. She also has allergic rhinitis, for which she uses nasal fluticasone. Yet despite this regimen, Ms. D reports she still experiences wheezing, chest tightness, and shortness of breath 3 to 4 times a week and is awak-ened by coughing at least twice a week. In the past 6 months, she has had one emergency department (ED) visit and completed 2 courses of oral steroids.
Ms. D has gained weight since her last visit 3 months ago; her body mass index has gone from 27.5 to 29 kg/m2. And, while she has always been somewhat anxious, Ms. D notes that her anxiety has gotten progressively worse, as well.
About 25 million Americans—approximately one in 12—suffer from asthma1 and, despite improvements in asthma guidelines and treatment in the last 20 years,2 many still struggle with uncontrolled symptoms.3 The consequences can be severe.
Suboptimal control of asthma is associated with a significant decrease in quality of life, a greater likelihood of absence from work or school, and an increased risk for life-threatening events, trips to the ED, hospital admissions, and death.1 A multifaceted approach, including regular assessment, aggressive medication management, and attention to comorbidities, is needed to alleviate the suffering of patients with persistent asthma. This evidence-based review can help you provide such broad-based treatment.
Diagnosis and classification go hand in hand
The cornerstones of asthma management are accurate diagnosis and assessment of disease severity, based on both qualitative and quantitative measures. Start with a patient history, eliciting information about symptoms, triggers, risk factors, and most importantly, how often symptoms occur. Classic high-pitched wheezing sounds during exhalation, a cough that often worsens at night, shortness of breath, and chest tightness should raise suspicion for an asthma diagnosis.2 But frequency (and timing) of symptoms and exacerbations, as well as changes in the patient’s ability to function normally, help to determine whether asthma is classified as mild intermittent, mild persistent, moderate persistent, or severe persistent (TABLE).2
TABLE
Classifying asthma severity2
| Findings | Mild intermittent | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|
| Frequency | ≤2/wk | >2/wk, but <1/d | Daily | Continuous |
| Exacerbations | Rare | <2/wk | ≥2/wk | Frequent |
| Activity level | Normal | May decrease with exacerbation | Frequently limited | Significantly limited |
| Nighttime symptoms | ≤2/mo | >2/mo | >1/wk | Frequent |
| FEV1 (or PEF) predicted | >80% | >80% | >60% to <80% | ≤60% |
| PEF variability | <20% | 20%-30% | >30% | >30% |
| FEV1, forced expiratory volume in one second; PEF, peak expiratory flow. | ||||
Because asthma treatment should be based on its classification, an accurate assessment of disease severity is especially important for patients like Ms. D, who have been treated for asthma but continue to have unresolved symptoms. Keep in mind that asthma classification should be based on the worst symptom a patient has, not necessarily the symptom that occurs most frequently. Thus, a patient who has daytime symptoms requiring use of a rescue inhaler 2 to 3 times a week but is awakened at night with shortness of breath 2 times a week would receive a diagnosis of moderate persistent asthma on the basis of the night-time symptoms.
In assessing asthma severity, it is also important to ask specifically about recent events, including ED visits, hospitalizations, and intubations. This information, as well as answers to questions about smoking status, mental health problems, quality of life, and treatment compliance—and whether the patient can afford to purchase the asthma medications you’ve prescribed—can be used to assess the likelihood of poor outcomes.2
Factor in spirometry findings
History and physical examination alone cannot adequately diagnose and classify asthma severity.4,5 Spirometry, a reimbursable office test that can be administered by trained staff members, can be beneficial for any patient older than 5 years for whom a diagnosis of asthma is being considered or disease severity being determined.2 Other objective measures, such as the Mini Asthma Quality of Life questionnaire (http://erj.ersjournals.com/content/14/1/32.full.pdf+html) and peak expiratory flow measurement, may be helpful, as well.2,6
Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
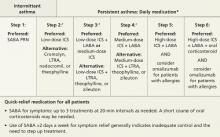
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.
A dose increase avoids both the additional risk of adverse drug reactions and the added cost associated with another medication. But the easiest solution is not necessarily the best. Consider the evidence detailed below, which includes findings from studies published after the NHLBI’s guidelines.
The research on LABAs
LABAs have been widely used as adjunctive therapy for adults with asthma. However, a 2006 study raised safety concerns.16
The Salmeterol Multicenter Asthma Research Trial (SMART) compared the safety of the LABA salmeterol with a placebo added to usual asthma care over a 28-week treatment period. Overall, the primary composite end point—the number of respiratory-related deaths or life-threatening events—was low, and not statistically significant for salmeterol (50 vs 36; relative risk [RR]=1.40; 95% confidence interval [CI], 0.91-2.14).16 However, individual outcomes—respiratory-related deaths, asthma-related deaths, and asthma-related deaths or life-threatening episodes—were significantly more likely in the salmeterol group compared with the placebo group. In subgroup analysis, African American patients were found to be at greatest risk.16
It is hard to draw general conclusions from these data because the study was terminated early and poor outcomes were limited to a particular study year. Nonetheless, many physicians remain wary of LABAs as adjunctive therapy because of these findings and the media publicity they generated.
A 2010 Cochrane review provided additional data on the safety and efficacy of the combination of a LABA and ICS compared with a higher dose of ICS.17 The review, which included 48 randomized controlled trials, found that combination therapy had a lower risk of exacerbations for which oral corticosteroids were required than a higher dose of ICS (RR=0.88; 95% CI, 0.78-0.98; P=.02). The median number needed to treat (NNT) was 73. No significant difference in the risk of overall adverse events (RR=0.99; 95% CI, 0.95-1.03) was found, but there was an increase in the risk of tremor (RR=1.84; 95% CI, 1.20-2.82) and a decrease in risk for oral thrush (RR=0.58; 95% CI, 0.40-0.86) in the combination therapy group.
While the Cochrane review did not show a combination of LABA and ICS to be less safe overall than higher doses of ICS alone, the findings were less favorable for children and patients with higher baseline lung function, in circumstances in which the combination therapy was taken for a longer duration, and when the LABA being studied was formoterol.17
Overall, it is when a LABA is delivered via separate inhaler that adverse outcomes have been reported. Findings have been positive when the LABA is combined with ICS, and this combination is recommended as maintenance therapy for moderate to severe asthma.
Two new studies, published in March 2013, reported successful use of a LABA-ICS combination not only for maintenance via scheduled dosing, but also for early phases of exacerbation via extra dosing—an approach called Single inhaler Maintenance and Reliever Therapy (SMART).18,19 In both studies, SMART resulted in less excessive use of SABAs and less need for oral steroids, fewer hospitalizations for asthma, and fewer cases of progression to a full-blown exacerbation.
The takeaway: LABAs should be reserved for use as an adjunct to ICS in adults with poor baseline pulmonary function tests or severe asthma, and delivered as a combination product with ICS, not as a separate inhaled medication. SMART is a safe and effective means of administering LABA-ICS therapy for some patients at risk for frequent severe exacerbations.
When to consider LTRAs
LTRAs can be valuable medications in asthma management and there are extensive data on their use, particularly in the treatment of children with asthma. A Cochrane review published in 2012, however, supported current guideline recommendations, finding that as monotherapy, ICS are superior to LTRAs.20
When LTRAs as an adjunctive therapy to ICS were compared with ICS monotherapy, researchers found a modest improvement in PEF (weighted mean difference [WMD] =7.7 L/min; 95% CI, 3.6-11.8) in the group receiving combination therapy and a decrease in the need for a SABA as rescue therapy (WMD=1 puff/week; 95% CI, 0.5-2.0).21 There was no significant reduction in the risk of exacerbations requiring systemic steroids (RR=0.64; 95% CI, 0.38-1.07).
LABAs and LTRAs go head to head. A 2010 Cochrane review compared the efficacy and safety of a daily LABA vs a LTRA as add-on therapy for patients whose asthma was not well controlled with ICS monotherapy.22 The LABA/ICS combination was significantly better at reducing the risk of exacerbations requiring systemic corticosteroids than monotherapy with either a LTRA or ICS, reducing the risk from 11% to 9% (RR=0.83; 95% CI, 0.71-0.97). The NNT to prevent one exacerbation over 48 weeks was 38 (95% CI, 22-244).22
The safety of LABAs continues to be a concern, however, as serious adverse events were more common in the LABA group. The number needed to harm (NNH) with LABA therapy vs LTRA over 48 weeks was 78; 95% CI, 33 to infinity.22 (The width of the CI indicates that while harm is possible in as few as 33 patients, it is also possible that an infinite number of patients would need to be treated for one individual to incur harm.) Overall, the evidence suggests that LABAs are superior add-on therapy to ICS for the treatment of uncontrolled asthma compared with LTRAs, but their use nonetheless requires caution and close monitoring in African American and pediatric patients.17
Is there a role for a long-acting anticholinergic inhaler?
Long-acting anticholinergic medication (LAAM)—tiotropium is the only drug in this class on the market, but there are others in clinical trials—is the mainstay of therapy for chronic obstructive pulmonary disease. This drug class was not widely available or studied as an asthma treatment when the NHLBI guidelines were drafted.
A 2010 study of tiotropium challenged the notion that there is no place for LAAMs in asthma therapy. Using a 3-way crossover design, the study compared the addition of tiotropium to ICS with a double dose of ICS or a LABA/ICS combination.23
The results suggest that LAAMs could be useful in treating uncontrolled asthma. Compared with the double dose of ICS, the tiotropium/ICS combination increased PEF by a mean difference of 25.8 L/min (P<.001) and resulted in a statistically significant improvement in the proportion of asthma control days, FEV1, and daily symptom scores.23 As an adjunctive treatment to ICS, tiotropium was not inferior to a LABA.
CASE After a detailed history, physical exam, and diagnostic testing, Ms. D was given a diagnosis of moderate persistent asthma. We recognized the need to step up her treatment. Prior to making any changes in her medication regimen, however, our team, which included a clinical pharmacist, observed her use of inhaled medications and verified that she was using the inhaler properly. We then initiated combination therapy, pairing a LABA and ICS.
Comorbidities complicate asthma management
Asthma management is often complicated by other uncontrolled coexisting medical problems. Common comorbidities that can affect asthma severity include allergic rhinitis, chronic sinusitis, gastroesophageal reflux disease (GERD), obesity, obstructive sleep apnea (OSA), mental health disorders, tobacco use, and hormonal disturbances.2
Allergic rhinitis. Allergic rhinitis has been associated with worse asthma control and a negative impact on quality of life, and the upper airway inflammation associated with it should be treated.24
Antihistamines and nasal steroids are the most effective medical management. Some patients with allergic rhinitis benefit from blood or skin allergy testing for confirmation or to aid in avoidance. Referral to an allergist may be necessary if symptoms are recalcitrant, a food allergy is in question, or the diagnosis is unclear.
GERD. Compared with the general population, patients with asthma have a much higher risk of GERD, although it is not always symptomatic. While results are inconsistent and difficult to predict, treating symptomatic GERD with acid-blocking medications can result in better asthma control for some patients. However, proton pump inhibitors should not be used to treat asthma symptoms in patients with asymptomatic GERD.25,26
Obesity and OSA. Weight loss can significantly improve asthma control, decrease medication use, and improve quality of life.27,28 Obese patients are less likely to respond to treatment with ICS.2 Weight loss also benefits those who suffer from OSA, which may contribute to airway hyperresponsiveness.29
Mental health disorders. Compared with the general population, patients with asthma are more likely to have depression, anxiety, and panic disorders.30 Diagnosis and treatment of these comorbid conditions can lead to better asthma management, increased medication adherence, decreased health care utilization—including fewer ED visits and hospitalizations—and a better quality of life.30
CASE We also addressed our patient’s comorbidities—weight gain, allergic rhinitis, and anxiety. The allergic rhinitis was already well-controlled with a nasal steroid, but we suspected a relationship between Ms. D’s weight gain and increasing anxiety associated with some recent life events. We suggested she see a counselor, and she agreed.
When the patient returned in 12 weeks, she reported that she hardly needed her rescue inhaler anymore and that she was managing her anxiety more effectively. She also told us that she had begun a low-fat dietary regimen, and we confirmed that she had already lost 5 pounds.
CORRESPONDENCE
Stephen A. Wilson, MD, MPH, FAAFP, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; wilsons2@upmc.edu
1. American Academy of Allergy, Asthma, and Immunology. Asthma statistics. Available at: http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx. Accessed March 7, 2012.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for diagnosis and management of asthma. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Centers for Disease Control and Prevention. National surveillance for asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54.
4. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children. Arch Pediatr Adolesc Med. 2006;160:844-850.
5. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children. Am J Respir Crit Care Med. 2004;170:426-432.
6. Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32-38.
7. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179-187.
8. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
9. Bateman ED, Hurd SS, Barnes PJ, et al. Global Strategy for Asthma Management and Prevention. Eur Respir J. 2008;31:143-178.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. February 2013. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 7, 2013.
11. AsthmaMD. Available at: http://www.asthmamd.org/#resources/iphone_chart.jpg. Accessed March 7, 2013.
12. Indiegogo. Huff & Puff. Available at: http://www.indiegogo.com/projects/the-best-asthma-education-app-in-the-world-period. Accessed March 7, 2013.
13. Vimukti Technologies Pvt Ltd. Peak flow calculator. Available at: http://appworld.blackberry.com/webstore/content/7615. Accessed March 7, 2013,
14. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;35:1068-1080.
15. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375-387.
16. Nelson HS, Weiss ST, Bleeker ER, et al. The Salmeterol Multicenter Asthma Research Trial. Chest. 2006;129:15-26.
17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533.-
18. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma. Lancet Respir Med. 2013;1:23-31.
19. Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerba-tions. Lancet Respir Med. 2013;1:32-42.
20. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.-
21. Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004;(1):CD003133.-
22. Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011;(5):CD003137.-
23. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
24. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65:1290-1297.
25. Gibson PG, Henry RL, Coughlan JL. Gastroesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003;(2):CD001496.-
26. The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1499.
27. Eneli IU, Skybo T, Camargo CA, Jr. Weight loss and asthma. Thorax. 2008;63:671-676.
28. Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma. BMJ. 2000;320:827-832.
29. Sariman N, Levent E, Cubuk R, et al. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath. 2011;15:341-50.
30. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22-28.
• Classify and treat asthma based on the patient’s worst symptom, whether or not it is the symptom that occurs most frequently. C
• Treat patients with poorly controlled asthma aggressively to gain quick control, then scale back slowly to the fewest medications and lowest doses needed to maintain control. A
• Reserve long-acting beta-agonists for use as an adjunct to inhaled corticosteroids for adults with poor baseline pulmonary function tests. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Angela D, a 34-year-old patient, has asthma with recurrent exacerbations. She uses a low-dose inhaled corticosteroid (ICS) daily and an albuterol inhaler, as needed, for shortness of breath or wheezing. She also has allergic rhinitis, for which she uses nasal fluticasone. Yet despite this regimen, Ms. D reports she still experiences wheezing, chest tightness, and shortness of breath 3 to 4 times a week and is awak-ened by coughing at least twice a week. In the past 6 months, she has had one emergency department (ED) visit and completed 2 courses of oral steroids.
Ms. D has gained weight since her last visit 3 months ago; her body mass index has gone from 27.5 to 29 kg/m2. And, while she has always been somewhat anxious, Ms. D notes that her anxiety has gotten progressively worse, as well.
About 25 million Americans—approximately one in 12—suffer from asthma1 and, despite improvements in asthma guidelines and treatment in the last 20 years,2 many still struggle with uncontrolled symptoms.3 The consequences can be severe.
Suboptimal control of asthma is associated with a significant decrease in quality of life, a greater likelihood of absence from work or school, and an increased risk for life-threatening events, trips to the ED, hospital admissions, and death.1 A multifaceted approach, including regular assessment, aggressive medication management, and attention to comorbidities, is needed to alleviate the suffering of patients with persistent asthma. This evidence-based review can help you provide such broad-based treatment.
Diagnosis and classification go hand in hand
The cornerstones of asthma management are accurate diagnosis and assessment of disease severity, based on both qualitative and quantitative measures. Start with a patient history, eliciting information about symptoms, triggers, risk factors, and most importantly, how often symptoms occur. Classic high-pitched wheezing sounds during exhalation, a cough that often worsens at night, shortness of breath, and chest tightness should raise suspicion for an asthma diagnosis.2 But frequency (and timing) of symptoms and exacerbations, as well as changes in the patient’s ability to function normally, help to determine whether asthma is classified as mild intermittent, mild persistent, moderate persistent, or severe persistent (TABLE).2
TABLE
Classifying asthma severity2
| Findings | Mild intermittent | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|
| Frequency | ≤2/wk | >2/wk, but <1/d | Daily | Continuous |
| Exacerbations | Rare | <2/wk | ≥2/wk | Frequent |
| Activity level | Normal | May decrease with exacerbation | Frequently limited | Significantly limited |
| Nighttime symptoms | ≤2/mo | >2/mo | >1/wk | Frequent |
| FEV1 (or PEF) predicted | >80% | >80% | >60% to <80% | ≤60% |
| PEF variability | <20% | 20%-30% | >30% | >30% |
| FEV1, forced expiratory volume in one second; PEF, peak expiratory flow. | ||||
Because asthma treatment should be based on its classification, an accurate assessment of disease severity is especially important for patients like Ms. D, who have been treated for asthma but continue to have unresolved symptoms. Keep in mind that asthma classification should be based on the worst symptom a patient has, not necessarily the symptom that occurs most frequently. Thus, a patient who has daytime symptoms requiring use of a rescue inhaler 2 to 3 times a week but is awakened at night with shortness of breath 2 times a week would receive a diagnosis of moderate persistent asthma on the basis of the night-time symptoms.
In assessing asthma severity, it is also important to ask specifically about recent events, including ED visits, hospitalizations, and intubations. This information, as well as answers to questions about smoking status, mental health problems, quality of life, and treatment compliance—and whether the patient can afford to purchase the asthma medications you’ve prescribed—can be used to assess the likelihood of poor outcomes.2
Factor in spirometry findings
History and physical examination alone cannot adequately diagnose and classify asthma severity.4,5 Spirometry, a reimbursable office test that can be administered by trained staff members, can be beneficial for any patient older than 5 years for whom a diagnosis of asthma is being considered or disease severity being determined.2 Other objective measures, such as the Mini Asthma Quality of Life questionnaire (http://erj.ersjournals.com/content/14/1/32.full.pdf+html) and peak expiratory flow measurement, may be helpful, as well.2,6
Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.
A dose increase avoids both the additional risk of adverse drug reactions and the added cost associated with another medication. But the easiest solution is not necessarily the best. Consider the evidence detailed below, which includes findings from studies published after the NHLBI’s guidelines.
The research on LABAs
LABAs have been widely used as adjunctive therapy for adults with asthma. However, a 2006 study raised safety concerns.16
The Salmeterol Multicenter Asthma Research Trial (SMART) compared the safety of the LABA salmeterol with a placebo added to usual asthma care over a 28-week treatment period. Overall, the primary composite end point—the number of respiratory-related deaths or life-threatening events—was low, and not statistically significant for salmeterol (50 vs 36; relative risk [RR]=1.40; 95% confidence interval [CI], 0.91-2.14).16 However, individual outcomes—respiratory-related deaths, asthma-related deaths, and asthma-related deaths or life-threatening episodes—were significantly more likely in the salmeterol group compared with the placebo group. In subgroup analysis, African American patients were found to be at greatest risk.16
It is hard to draw general conclusions from these data because the study was terminated early and poor outcomes were limited to a particular study year. Nonetheless, many physicians remain wary of LABAs as adjunctive therapy because of these findings and the media publicity they generated.
A 2010 Cochrane review provided additional data on the safety and efficacy of the combination of a LABA and ICS compared with a higher dose of ICS.17 The review, which included 48 randomized controlled trials, found that combination therapy had a lower risk of exacerbations for which oral corticosteroids were required than a higher dose of ICS (RR=0.88; 95% CI, 0.78-0.98; P=.02). The median number needed to treat (NNT) was 73. No significant difference in the risk of overall adverse events (RR=0.99; 95% CI, 0.95-1.03) was found, but there was an increase in the risk of tremor (RR=1.84; 95% CI, 1.20-2.82) and a decrease in risk for oral thrush (RR=0.58; 95% CI, 0.40-0.86) in the combination therapy group.
While the Cochrane review did not show a combination of LABA and ICS to be less safe overall than higher doses of ICS alone, the findings were less favorable for children and patients with higher baseline lung function, in circumstances in which the combination therapy was taken for a longer duration, and when the LABA being studied was formoterol.17
Overall, it is when a LABA is delivered via separate inhaler that adverse outcomes have been reported. Findings have been positive when the LABA is combined with ICS, and this combination is recommended as maintenance therapy for moderate to severe asthma.
Two new studies, published in March 2013, reported successful use of a LABA-ICS combination not only for maintenance via scheduled dosing, but also for early phases of exacerbation via extra dosing—an approach called Single inhaler Maintenance and Reliever Therapy (SMART).18,19 In both studies, SMART resulted in less excessive use of SABAs and less need for oral steroids, fewer hospitalizations for asthma, and fewer cases of progression to a full-blown exacerbation.
The takeaway: LABAs should be reserved for use as an adjunct to ICS in adults with poor baseline pulmonary function tests or severe asthma, and delivered as a combination product with ICS, not as a separate inhaled medication. SMART is a safe and effective means of administering LABA-ICS therapy for some patients at risk for frequent severe exacerbations.
When to consider LTRAs
LTRAs can be valuable medications in asthma management and there are extensive data on their use, particularly in the treatment of children with asthma. A Cochrane review published in 2012, however, supported current guideline recommendations, finding that as monotherapy, ICS are superior to LTRAs.20
When LTRAs as an adjunctive therapy to ICS were compared with ICS monotherapy, researchers found a modest improvement in PEF (weighted mean difference [WMD] =7.7 L/min; 95% CI, 3.6-11.8) in the group receiving combination therapy and a decrease in the need for a SABA as rescue therapy (WMD=1 puff/week; 95% CI, 0.5-2.0).21 There was no significant reduction in the risk of exacerbations requiring systemic steroids (RR=0.64; 95% CI, 0.38-1.07).
LABAs and LTRAs go head to head. A 2010 Cochrane review compared the efficacy and safety of a daily LABA vs a LTRA as add-on therapy for patients whose asthma was not well controlled with ICS monotherapy.22 The LABA/ICS combination was significantly better at reducing the risk of exacerbations requiring systemic corticosteroids than monotherapy with either a LTRA or ICS, reducing the risk from 11% to 9% (RR=0.83; 95% CI, 0.71-0.97). The NNT to prevent one exacerbation over 48 weeks was 38 (95% CI, 22-244).22
The safety of LABAs continues to be a concern, however, as serious adverse events were more common in the LABA group. The number needed to harm (NNH) with LABA therapy vs LTRA over 48 weeks was 78; 95% CI, 33 to infinity.22 (The width of the CI indicates that while harm is possible in as few as 33 patients, it is also possible that an infinite number of patients would need to be treated for one individual to incur harm.) Overall, the evidence suggests that LABAs are superior add-on therapy to ICS for the treatment of uncontrolled asthma compared with LTRAs, but their use nonetheless requires caution and close monitoring in African American and pediatric patients.17
Is there a role for a long-acting anticholinergic inhaler?
Long-acting anticholinergic medication (LAAM)—tiotropium is the only drug in this class on the market, but there are others in clinical trials—is the mainstay of therapy for chronic obstructive pulmonary disease. This drug class was not widely available or studied as an asthma treatment when the NHLBI guidelines were drafted.
A 2010 study of tiotropium challenged the notion that there is no place for LAAMs in asthma therapy. Using a 3-way crossover design, the study compared the addition of tiotropium to ICS with a double dose of ICS or a LABA/ICS combination.23
The results suggest that LAAMs could be useful in treating uncontrolled asthma. Compared with the double dose of ICS, the tiotropium/ICS combination increased PEF by a mean difference of 25.8 L/min (P<.001) and resulted in a statistically significant improvement in the proportion of asthma control days, FEV1, and daily symptom scores.23 As an adjunctive treatment to ICS, tiotropium was not inferior to a LABA.
CASE After a detailed history, physical exam, and diagnostic testing, Ms. D was given a diagnosis of moderate persistent asthma. We recognized the need to step up her treatment. Prior to making any changes in her medication regimen, however, our team, which included a clinical pharmacist, observed her use of inhaled medications and verified that she was using the inhaler properly. We then initiated combination therapy, pairing a LABA and ICS.
Comorbidities complicate asthma management
Asthma management is often complicated by other uncontrolled coexisting medical problems. Common comorbidities that can affect asthma severity include allergic rhinitis, chronic sinusitis, gastroesophageal reflux disease (GERD), obesity, obstructive sleep apnea (OSA), mental health disorders, tobacco use, and hormonal disturbances.2
Allergic rhinitis. Allergic rhinitis has been associated with worse asthma control and a negative impact on quality of life, and the upper airway inflammation associated with it should be treated.24
Antihistamines and nasal steroids are the most effective medical management. Some patients with allergic rhinitis benefit from blood or skin allergy testing for confirmation or to aid in avoidance. Referral to an allergist may be necessary if symptoms are recalcitrant, a food allergy is in question, or the diagnosis is unclear.
GERD. Compared with the general population, patients with asthma have a much higher risk of GERD, although it is not always symptomatic. While results are inconsistent and difficult to predict, treating symptomatic GERD with acid-blocking medications can result in better asthma control for some patients. However, proton pump inhibitors should not be used to treat asthma symptoms in patients with asymptomatic GERD.25,26
Obesity and OSA. Weight loss can significantly improve asthma control, decrease medication use, and improve quality of life.27,28 Obese patients are less likely to respond to treatment with ICS.2 Weight loss also benefits those who suffer from OSA, which may contribute to airway hyperresponsiveness.29
Mental health disorders. Compared with the general population, patients with asthma are more likely to have depression, anxiety, and panic disorders.30 Diagnosis and treatment of these comorbid conditions can lead to better asthma management, increased medication adherence, decreased health care utilization—including fewer ED visits and hospitalizations—and a better quality of life.30
CASE We also addressed our patient’s comorbidities—weight gain, allergic rhinitis, and anxiety. The allergic rhinitis was already well-controlled with a nasal steroid, but we suspected a relationship between Ms. D’s weight gain and increasing anxiety associated with some recent life events. We suggested she see a counselor, and she agreed.
When the patient returned in 12 weeks, she reported that she hardly needed her rescue inhaler anymore and that she was managing her anxiety more effectively. She also told us that she had begun a low-fat dietary regimen, and we confirmed that she had already lost 5 pounds.
CORRESPONDENCE
Stephen A. Wilson, MD, MPH, FAAFP, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; wilsons2@upmc.edu
• Classify and treat asthma based on the patient’s worst symptom, whether or not it is the symptom that occurs most frequently. C
• Treat patients with poorly controlled asthma aggressively to gain quick control, then scale back slowly to the fewest medications and lowest doses needed to maintain control. A
• Reserve long-acting beta-agonists for use as an adjunct to inhaled corticosteroids for adults with poor baseline pulmonary function tests. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Angela D, a 34-year-old patient, has asthma with recurrent exacerbations. She uses a low-dose inhaled corticosteroid (ICS) daily and an albuterol inhaler, as needed, for shortness of breath or wheezing. She also has allergic rhinitis, for which she uses nasal fluticasone. Yet despite this regimen, Ms. D reports she still experiences wheezing, chest tightness, and shortness of breath 3 to 4 times a week and is awak-ened by coughing at least twice a week. In the past 6 months, she has had one emergency department (ED) visit and completed 2 courses of oral steroids.
Ms. D has gained weight since her last visit 3 months ago; her body mass index has gone from 27.5 to 29 kg/m2. And, while she has always been somewhat anxious, Ms. D notes that her anxiety has gotten progressively worse, as well.
About 25 million Americans—approximately one in 12—suffer from asthma1 and, despite improvements in asthma guidelines and treatment in the last 20 years,2 many still struggle with uncontrolled symptoms.3 The consequences can be severe.
Suboptimal control of asthma is associated with a significant decrease in quality of life, a greater likelihood of absence from work or school, and an increased risk for life-threatening events, trips to the ED, hospital admissions, and death.1 A multifaceted approach, including regular assessment, aggressive medication management, and attention to comorbidities, is needed to alleviate the suffering of patients with persistent asthma. This evidence-based review can help you provide such broad-based treatment.
Diagnosis and classification go hand in hand
The cornerstones of asthma management are accurate diagnosis and assessment of disease severity, based on both qualitative and quantitative measures. Start with a patient history, eliciting information about symptoms, triggers, risk factors, and most importantly, how often symptoms occur. Classic high-pitched wheezing sounds during exhalation, a cough that often worsens at night, shortness of breath, and chest tightness should raise suspicion for an asthma diagnosis.2 But frequency (and timing) of symptoms and exacerbations, as well as changes in the patient’s ability to function normally, help to determine whether asthma is classified as mild intermittent, mild persistent, moderate persistent, or severe persistent (TABLE).2
TABLE
Classifying asthma severity2
| Findings | Mild intermittent | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|
| Frequency | ≤2/wk | >2/wk, but <1/d | Daily | Continuous |
| Exacerbations | Rare | <2/wk | ≥2/wk | Frequent |
| Activity level | Normal | May decrease with exacerbation | Frequently limited | Significantly limited |
| Nighttime symptoms | ≤2/mo | >2/mo | >1/wk | Frequent |
| FEV1 (or PEF) predicted | >80% | >80% | >60% to <80% | ≤60% |
| PEF variability | <20% | 20%-30% | >30% | >30% |
| FEV1, forced expiratory volume in one second; PEF, peak expiratory flow. | ||||
Because asthma treatment should be based on its classification, an accurate assessment of disease severity is especially important for patients like Ms. D, who have been treated for asthma but continue to have unresolved symptoms. Keep in mind that asthma classification should be based on the worst symptom a patient has, not necessarily the symptom that occurs most frequently. Thus, a patient who has daytime symptoms requiring use of a rescue inhaler 2 to 3 times a week but is awakened at night with shortness of breath 2 times a week would receive a diagnosis of moderate persistent asthma on the basis of the night-time symptoms.
In assessing asthma severity, it is also important to ask specifically about recent events, including ED visits, hospitalizations, and intubations. This information, as well as answers to questions about smoking status, mental health problems, quality of life, and treatment compliance—and whether the patient can afford to purchase the asthma medications you’ve prescribed—can be used to assess the likelihood of poor outcomes.2
Factor in spirometry findings
History and physical examination alone cannot adequately diagnose and classify asthma severity.4,5 Spirometry, a reimbursable office test that can be administered by trained staff members, can be beneficial for any patient older than 5 years for whom a diagnosis of asthma is being considered or disease severity being determined.2 Other objective measures, such as the Mini Asthma Quality of Life questionnaire (http://erj.ersjournals.com/content/14/1/32.full.pdf+html) and peak expiratory flow measurement, may be helpful, as well.2,6
Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.
A dose increase avoids both the additional risk of adverse drug reactions and the added cost associated with another medication. But the easiest solution is not necessarily the best. Consider the evidence detailed below, which includes findings from studies published after the NHLBI’s guidelines.
The research on LABAs
LABAs have been widely used as adjunctive therapy for adults with asthma. However, a 2006 study raised safety concerns.16
The Salmeterol Multicenter Asthma Research Trial (SMART) compared the safety of the LABA salmeterol with a placebo added to usual asthma care over a 28-week treatment period. Overall, the primary composite end point—the number of respiratory-related deaths or life-threatening events—was low, and not statistically significant for salmeterol (50 vs 36; relative risk [RR]=1.40; 95% confidence interval [CI], 0.91-2.14).16 However, individual outcomes—respiratory-related deaths, asthma-related deaths, and asthma-related deaths or life-threatening episodes—were significantly more likely in the salmeterol group compared with the placebo group. In subgroup analysis, African American patients were found to be at greatest risk.16
It is hard to draw general conclusions from these data because the study was terminated early and poor outcomes were limited to a particular study year. Nonetheless, many physicians remain wary of LABAs as adjunctive therapy because of these findings and the media publicity they generated.
A 2010 Cochrane review provided additional data on the safety and efficacy of the combination of a LABA and ICS compared with a higher dose of ICS.17 The review, which included 48 randomized controlled trials, found that combination therapy had a lower risk of exacerbations for which oral corticosteroids were required than a higher dose of ICS (RR=0.88; 95% CI, 0.78-0.98; P=.02). The median number needed to treat (NNT) was 73. No significant difference in the risk of overall adverse events (RR=0.99; 95% CI, 0.95-1.03) was found, but there was an increase in the risk of tremor (RR=1.84; 95% CI, 1.20-2.82) and a decrease in risk for oral thrush (RR=0.58; 95% CI, 0.40-0.86) in the combination therapy group.
While the Cochrane review did not show a combination of LABA and ICS to be less safe overall than higher doses of ICS alone, the findings were less favorable for children and patients with higher baseline lung function, in circumstances in which the combination therapy was taken for a longer duration, and when the LABA being studied was formoterol.17
Overall, it is when a LABA is delivered via separate inhaler that adverse outcomes have been reported. Findings have been positive when the LABA is combined with ICS, and this combination is recommended as maintenance therapy for moderate to severe asthma.
Two new studies, published in March 2013, reported successful use of a LABA-ICS combination not only for maintenance via scheduled dosing, but also for early phases of exacerbation via extra dosing—an approach called Single inhaler Maintenance and Reliever Therapy (SMART).18,19 In both studies, SMART resulted in less excessive use of SABAs and less need for oral steroids, fewer hospitalizations for asthma, and fewer cases of progression to a full-blown exacerbation.
The takeaway: LABAs should be reserved for use as an adjunct to ICS in adults with poor baseline pulmonary function tests or severe asthma, and delivered as a combination product with ICS, not as a separate inhaled medication. SMART is a safe and effective means of administering LABA-ICS therapy for some patients at risk for frequent severe exacerbations.
When to consider LTRAs
LTRAs can be valuable medications in asthma management and there are extensive data on their use, particularly in the treatment of children with asthma. A Cochrane review published in 2012, however, supported current guideline recommendations, finding that as monotherapy, ICS are superior to LTRAs.20
When LTRAs as an adjunctive therapy to ICS were compared with ICS monotherapy, researchers found a modest improvement in PEF (weighted mean difference [WMD] =7.7 L/min; 95% CI, 3.6-11.8) in the group receiving combination therapy and a decrease in the need for a SABA as rescue therapy (WMD=1 puff/week; 95% CI, 0.5-2.0).21 There was no significant reduction in the risk of exacerbations requiring systemic steroids (RR=0.64; 95% CI, 0.38-1.07).
LABAs and LTRAs go head to head. A 2010 Cochrane review compared the efficacy and safety of a daily LABA vs a LTRA as add-on therapy for patients whose asthma was not well controlled with ICS monotherapy.22 The LABA/ICS combination was significantly better at reducing the risk of exacerbations requiring systemic corticosteroids than monotherapy with either a LTRA or ICS, reducing the risk from 11% to 9% (RR=0.83; 95% CI, 0.71-0.97). The NNT to prevent one exacerbation over 48 weeks was 38 (95% CI, 22-244).22
The safety of LABAs continues to be a concern, however, as serious adverse events were more common in the LABA group. The number needed to harm (NNH) with LABA therapy vs LTRA over 48 weeks was 78; 95% CI, 33 to infinity.22 (The width of the CI indicates that while harm is possible in as few as 33 patients, it is also possible that an infinite number of patients would need to be treated for one individual to incur harm.) Overall, the evidence suggests that LABAs are superior add-on therapy to ICS for the treatment of uncontrolled asthma compared with LTRAs, but their use nonetheless requires caution and close monitoring in African American and pediatric patients.17
Is there a role for a long-acting anticholinergic inhaler?
Long-acting anticholinergic medication (LAAM)—tiotropium is the only drug in this class on the market, but there are others in clinical trials—is the mainstay of therapy for chronic obstructive pulmonary disease. This drug class was not widely available or studied as an asthma treatment when the NHLBI guidelines were drafted.
A 2010 study of tiotropium challenged the notion that there is no place for LAAMs in asthma therapy. Using a 3-way crossover design, the study compared the addition of tiotropium to ICS with a double dose of ICS or a LABA/ICS combination.23
The results suggest that LAAMs could be useful in treating uncontrolled asthma. Compared with the double dose of ICS, the tiotropium/ICS combination increased PEF by a mean difference of 25.8 L/min (P<.001) and resulted in a statistically significant improvement in the proportion of asthma control days, FEV1, and daily symptom scores.23 As an adjunctive treatment to ICS, tiotropium was not inferior to a LABA.
CASE After a detailed history, physical exam, and diagnostic testing, Ms. D was given a diagnosis of moderate persistent asthma. We recognized the need to step up her treatment. Prior to making any changes in her medication regimen, however, our team, which included a clinical pharmacist, observed her use of inhaled medications and verified that she was using the inhaler properly. We then initiated combination therapy, pairing a LABA and ICS.
Comorbidities complicate asthma management
Asthma management is often complicated by other uncontrolled coexisting medical problems. Common comorbidities that can affect asthma severity include allergic rhinitis, chronic sinusitis, gastroesophageal reflux disease (GERD), obesity, obstructive sleep apnea (OSA), mental health disorders, tobacco use, and hormonal disturbances.2
Allergic rhinitis. Allergic rhinitis has been associated with worse asthma control and a negative impact on quality of life, and the upper airway inflammation associated with it should be treated.24
Antihistamines and nasal steroids are the most effective medical management. Some patients with allergic rhinitis benefit from blood or skin allergy testing for confirmation or to aid in avoidance. Referral to an allergist may be necessary if symptoms are recalcitrant, a food allergy is in question, or the diagnosis is unclear.
GERD. Compared with the general population, patients with asthma have a much higher risk of GERD, although it is not always symptomatic. While results are inconsistent and difficult to predict, treating symptomatic GERD with acid-blocking medications can result in better asthma control for some patients. However, proton pump inhibitors should not be used to treat asthma symptoms in patients with asymptomatic GERD.25,26
Obesity and OSA. Weight loss can significantly improve asthma control, decrease medication use, and improve quality of life.27,28 Obese patients are less likely to respond to treatment with ICS.2 Weight loss also benefits those who suffer from OSA, which may contribute to airway hyperresponsiveness.29
Mental health disorders. Compared with the general population, patients with asthma are more likely to have depression, anxiety, and panic disorders.30 Diagnosis and treatment of these comorbid conditions can lead to better asthma management, increased medication adherence, decreased health care utilization—including fewer ED visits and hospitalizations—and a better quality of life.30
CASE We also addressed our patient’s comorbidities—weight gain, allergic rhinitis, and anxiety. The allergic rhinitis was already well-controlled with a nasal steroid, but we suspected a relationship between Ms. D’s weight gain and increasing anxiety associated with some recent life events. We suggested she see a counselor, and she agreed.
When the patient returned in 12 weeks, she reported that she hardly needed her rescue inhaler anymore and that she was managing her anxiety more effectively. She also told us that she had begun a low-fat dietary regimen, and we confirmed that she had already lost 5 pounds.
CORRESPONDENCE
Stephen A. Wilson, MD, MPH, FAAFP, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; wilsons2@upmc.edu
1. American Academy of Allergy, Asthma, and Immunology. Asthma statistics. Available at: http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx. Accessed March 7, 2012.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for diagnosis and management of asthma. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Centers for Disease Control and Prevention. National surveillance for asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54.
4. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children. Arch Pediatr Adolesc Med. 2006;160:844-850.
5. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children. Am J Respir Crit Care Med. 2004;170:426-432.
6. Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32-38.
7. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179-187.
8. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
9. Bateman ED, Hurd SS, Barnes PJ, et al. Global Strategy for Asthma Management and Prevention. Eur Respir J. 2008;31:143-178.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. February 2013. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 7, 2013.
11. AsthmaMD. Available at: http://www.asthmamd.org/#resources/iphone_chart.jpg. Accessed March 7, 2013.
12. Indiegogo. Huff & Puff. Available at: http://www.indiegogo.com/projects/the-best-asthma-education-app-in-the-world-period. Accessed March 7, 2013.
13. Vimukti Technologies Pvt Ltd. Peak flow calculator. Available at: http://appworld.blackberry.com/webstore/content/7615. Accessed March 7, 2013,
14. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;35:1068-1080.
15. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375-387.
16. Nelson HS, Weiss ST, Bleeker ER, et al. The Salmeterol Multicenter Asthma Research Trial. Chest. 2006;129:15-26.
17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533.-
18. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma. Lancet Respir Med. 2013;1:23-31.
19. Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerba-tions. Lancet Respir Med. 2013;1:32-42.
20. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.-
21. Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004;(1):CD003133.-
22. Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011;(5):CD003137.-
23. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
24. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65:1290-1297.
25. Gibson PG, Henry RL, Coughlan JL. Gastroesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003;(2):CD001496.-
26. The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1499.
27. Eneli IU, Skybo T, Camargo CA, Jr. Weight loss and asthma. Thorax. 2008;63:671-676.
28. Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma. BMJ. 2000;320:827-832.
29. Sariman N, Levent E, Cubuk R, et al. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath. 2011;15:341-50.
30. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22-28.
1. American Academy of Allergy, Asthma, and Immunology. Asthma statistics. Available at: http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx. Accessed March 7, 2012.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for diagnosis and management of asthma. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Centers for Disease Control and Prevention. National surveillance for asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54.
4. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children. Arch Pediatr Adolesc Med. 2006;160:844-850.
5. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children. Am J Respir Crit Care Med. 2004;170:426-432.
6. Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32-38.
7. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179-187.
8. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
9. Bateman ED, Hurd SS, Barnes PJ, et al. Global Strategy for Asthma Management and Prevention. Eur Respir J. 2008;31:143-178.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. February 2013. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 7, 2013.
11. AsthmaMD. Available at: http://www.asthmamd.org/#resources/iphone_chart.jpg. Accessed March 7, 2013.
12. Indiegogo. Huff & Puff. Available at: http://www.indiegogo.com/projects/the-best-asthma-education-app-in-the-world-period. Accessed March 7, 2013.
13. Vimukti Technologies Pvt Ltd. Peak flow calculator. Available at: http://appworld.blackberry.com/webstore/content/7615. Accessed March 7, 2013,
14. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;35:1068-1080.
15. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375-387.
16. Nelson HS, Weiss ST, Bleeker ER, et al. The Salmeterol Multicenter Asthma Research Trial. Chest. 2006;129:15-26.
17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533.-
18. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma. Lancet Respir Med. 2013;1:23-31.
19. Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerba-tions. Lancet Respir Med. 2013;1:32-42.
20. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.-
21. Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004;(1):CD003133.-
22. Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011;(5):CD003137.-
23. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
24. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65:1290-1297.
25. Gibson PG, Henry RL, Coughlan JL. Gastroesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003;(2):CD001496.-
26. The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1499.
27. Eneli IU, Skybo T, Camargo CA, Jr. Weight loss and asthma. Thorax. 2008;63:671-676.
28. Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma. BMJ. 2000;320:827-832.
29. Sariman N, Levent E, Cubuk R, et al. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath. 2011;15:341-50.
30. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22-28.