User login
Gadolinium (Gd)-based contrast agents are frequently used in health care for enhancing magnetic resonance image (MRI) signals at low concentrations. Contrary to popular opinion, this widely used heavy metal is not biologically inert. Once notable for its safety profile, there is mounting evidence for Gd deposition in various organ systems of the body, even in those with normal renal function. A large knowledge gap remains concerning the potential harms of Gd deposition and the factors determining its elimination from the body. However, the findings of deposited Gd throughout various organs and their intracellular compartments even years after the initial exposure have been established. Here, we describe a case of a Vietnam-era veteran whose presentation, clinical, and laboratory findings were consistent within the spectrum of Gd deposition disease.
Case Presentation
A Vietnam-era veteran aged > 70 years presented for evaluation of Gd-based contrast agent–induced chronic multisymptomatic illness His medical history was significant for chronic low back pain, chronic hypertension, type 2 diabetes mellitus, and hypogonadism. Surgical history was notable for back surgery (24 years prior), laminectomy (2 years prior), shoulder replacement (2 years prior), and an epidural complicated by a hematoma (1 year prior). His presenting concerns included a painful and pruritic rash that worsened with showering, pain originating at the right Achilles tendon with migration to the knee, and shoulder pain. His symptoms started shortly after receiving multiple exposures to Gd-based contrast agents to enhance MRIs during his clinical care (Omniscan 20 mL, Omniscan 20 mL, and Gadovist 10 mL, administered 578, 565, and 496 days prior to the clinic visit, respectively). New onset headaches coincided with the timeline of symptom onset, in addition to hoarseness and liberation of an “oily substance” from the skin. More than one year prior to this clinic visit, he was considered for having polymyalgia rheumatica given the ambiguity of symptoms. Functional status remained impaired despite treatment with prednisone and methotrexate.
The patient’s military service was in the mid-1960s. He was deployed to Japan and had no knowledge of an Agent Orange exposure. His tobacco history was distant, and he reported no tattoos, prior transfusions, or occupational metal exposure
Clinical Findings
The patient was afebrile, normotensive (146/88 mmHg), and normocardic. His weight was 100 kg. He was well nourished and in no acute distress. The thought process was attentive, and his affect pleasant. Ocular examination was notable for arcus senilus. The fundoscopic examination was limited on the left, but there was no neovascularization on the right. Jugular venous pulsation was normal at 8 cm. Right ventricular impulse was slightly hyperdynamic, the rhythm was regular, and there was no abnormal splitting of S2. A soft-grade I/VI crescendo/decrescendo murmur was auscultated along the apex. Radial pulses were 2/2. He was not in respiratory distress, with equally resonant fields bilaterally. Lung sounds were clear bilaterally. A papular, erythematous rash was present in a general distribution over the chest, with few telangiectasias and some varicosity along his left arm. The skin had normal elasticity, although the skin of the hands and legs was papyraceous.
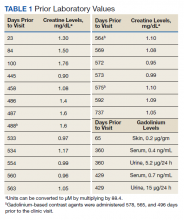
Gd levels were measured in the blood and urine (Table 1). Gd was detectable in the skin (0.2 µg/g) nearly 400 days after the last exposure. Gd was still detectable in the patient’s blood and urine (0.2 ng/mL and 0.5 µg/24 h, respectively) more than 3 years after his last exposure.
Discussion
In the United States, there are 40.44 MRI units per million people and 40 million MRIs are conducted annually. From 30 to 50% of these are enhanced with Gd-based contrast agents. In the past 30 years, there have been > 450 million contrast-enhanced MRI procedures.1
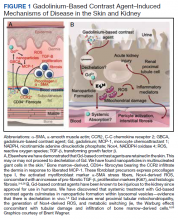
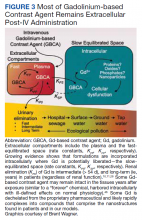
Gd is a rare earth metal. Among commercially available elements Gd has exceptional properties for enhancing MRI signals at low concentrations.1 The nonphysiologic metal is detoxified by chelation with proprietary multidentate formulations that enhance (primarily renal) elimination while retaining the paramagnetic and chemical properties for imaging. Gd exposure was found to be associated to iatrogenic nephrogenic systemic fibrosis in 2006 and later confirmed via multiple systematic reviews.2 Gd is retained in every vital organ after exposure.3 Gd-based contrast agents stimulate bone marrow–derived fibrocytes in mediating fibrosis, and bone marrow develop a memory of prior contrast exposure (Figure 1).4-6 Systemic fibrosis is mediated by the monocyte chemoattractant protein 1/C-C chemokine receptor 2.6,7 Even in the setting of normal renal function, Gd-based contrast induces the formation of Gd-rich nanoparticles in the skin and kidney.7,8 Far from being inert, Gd-based contrast agents induce systemic metabolic changes such as hypertriglyceridemia, elevations in low-density lipoprotein cholesterol, insulin resistance, and the Warburg effect (glycolytic/energy switching) in the renal cortex concomitant with profound mitochondrial abnormalities.8
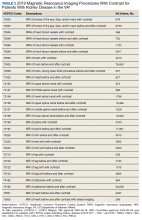
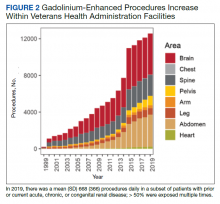
We have discovered that the rate of Gd-enhanced procedures has increased immensely within the Veterans Health Administration (VHA) system in a subset of patients with designated kidney disease (Table 2). Although a substantial number of procedures are dedicated to head and brain imaging within the VHA, the indications for Gd-enhanced diagnoses (eg, cardiac) are increasing (Figure 2).
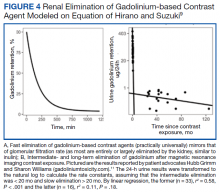
Retention of Gd can be modeled as a function of time (t) by the half-lives of the fast, intermediate, and slow phases of elimination (Ta, Tb, and Tc, respectively):9
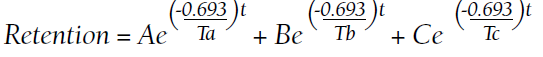
A, B, and C are the proportions (adding to 100%) that represent each of the compartments:
Numerous patients with normal renal function developed similar or novel symptoms that have been attributed to Gd concomitant with detectable urinary Gd years after exposure.11 Gd-based contrast agents are increasingly associated with cutaneous abnormalities even outside of nephrogenic systemic fibrosis. Gd-associated plaques develop in patients without kidney disease—these range from asymptomatic, pruritic, to burning.13 Histologic specimens reveal CD68 and factor XIIIa–positive spindle-shaped myeloid cells (the same mediators of iatrogenic systemic fibrosis) or CD34-positive cells. CD68 and factor XIIIa are distinctive for histologic specimens from patients with systemic fibrosis, and these markers have been detected in our preclinical models that demonstrated that bone marrow–derived cells are involved in mediating fibrosis.3,4,14-19 Similarly, CD34-positive cells have been historically associated with systemic fibrosis lesions.15,16,18-23 Plump osteocyte-appearing cells have also been noted (note that extraosseous metaplasia makes the histologic diagnosis of systemic fibrosis).14 Nephrogenic systemic fibrosis is an iatrogenic disease that can manifest years after exposure to Gd.5 Gd induces the recruitment of bone marrow–derived cells to the affected sites.4
The VA Health Service Research and Development Evidence Synthesis Program reviewed the safety of Gd-based contrast agents in patients with impaired kidney function.24,25 The group found only a single study of Gd and veterans. “Awareness and concern are growing about the long-term deposition of gadolinium in [the] brain and other tissues among patients with normal kidney function,” according to Lunyera and colleagues.25 The largest knowledge gap was that a comprehensive review “of all potential harms associated with gadolinium exposure” was not addressed. Furthermore, the group advised “caution in the use of [Gd-based contrast agents] in patients with severely impaired kidney function and acute kidney injury remains prudent, because the exact clinical factors contributing to [nephrogenic systemic fibrosis] risk in these subpopulations are still unknown.”25
Gd-based contrast agents—contrary to a widely held misconception—are not biologically inert.1 Gd-based contrast agents have a long history of association with acute renal injury. We have demonstrated that systemic treatment with MRI contrast agents leads to vacuolization of the proximal tubule and tubular injury.7,8 Kidney injury may be mediated by the generation of reactive oxygen species from NADPH oxidase 4 (Nox4).26
Gd retention, Gd-induced multisymptomatic illnesses, Gd-associated plaques, Gd-induced neurotoxicity, and nephrogenic systemic fibrosis are part of a continuum (with Gd as the common thread)—a theme of the September 8, 2017, US Food and Drug Administration (FDA) Medical Imaging Drugs Advisory Committee meeting.27 Patients, patient advocacy groups, and regulating agencies are concerned about long-term retention of a nonphysiologic rare earth element such as Gd.28-30 A patient advocacy group, The Lighthouse Project, collected information from patients linking the last date of Gd-based contrast agent exposure and urinary Gd.11 Data from their report suggest that the rate constants (valuable for the elimination equation above) are obtainable from 24-hour urine collections. Conceptually, Gd-induced diseases may represent a continuum that results from the retention of a nonphysiologic, toxic heavy rare earth metal.
As a heavy metal, Gd is not a natural physiologic trace element. Similar to numerous nonphysiologic metals, Gd is toxic. Inhaled Gd oxide (Gd2O3) dust leads to a number of time-dependent pathologies. Animal lung studies demonstrate reduced elasticity, enlarged cells, thickened lung walls, and recruitment of immune cells.31 Symptoms of acute IV Gd toxicity include decreased respiration, lethargy, abdominal cramps, and diarrhea.32 Pharmacologically, Gd concentrates in the liver and kidney and accumulates in the bone.32 Animals demonstrate intestinal depression and low blood pressure in response to Gd and, with higher doses, cardiovascular collapse.32 IV Gd chloride leads to metal deposition in the small blood vessels diffusely throughout the body, particularly in the lung and kidney and the metal is absorbed by the scavenging white blood cells.33 Gd chloride induces severe damage to the liver, spleen, and the digestive tract.33 Furthermore, this form of the toxicant metal markedly impacted functions associated with bleeding and clotting, ie, decreased platelet numbers and an increase in the laboratory-measured coagulation parameters.33 Semelka and colleagues have characterized chronic symptoms attributed to Gd-based contrast agents (not limited to chronic pain, headache, bone pain, skin thickening, and clouded mentation).34,35 Because Gd-induced conditions are underrecognized and ill-defined, disinherited patients often resort to untested (and potentially dangerous) chelation therapies.36
This patient presented with numerous symptoms that arose after Gd exposure. It is well established that Gd-based contrast agents (of any class) are retained in multiple organs (including the brain), for months to years. Gd-based contrast agents enter the cerebrospinal fluid within minutes of IV administration.37 Gd was found in the cerebrospinal fluid 9 months after administration in a case presented to the FDA Medical Imaging Drugs Advisory Committee.38 We know from intentional and accidental intrathecal administrations that Gd-based contrast agents are neurotoxic.39 Runge and colleagues demonstrated that Gd-based contrast agents exert mitochondrial toxicity in cultured neurons in vitro.40 McDonald and his team found Gd-rich nanoparticles within the brain neurons (cytoplasm and nuclei) from patients exposed to MRI contrast in the normal course of care.41 These nanoparticles are similar to what we have found in rodent models of Gd-induced disease.7,8,42
Prolonged elimination of Gd after MRI contrast administration (months to years) may be universal.10 Gd compartmentalizes into leukocytes and erythrocytes and into the cerebrospinal fluid within minutes.37,43 Patients with multisymptomatic illnesses attributed to Gd (Gd deposition disease) have perturbations in cytokine levels, many inflammatory.44,45 The results are concerning: Gd is retained intracellularly in vital organs, including brain neurons. It is inarguable that Gd is an alien, nonphysiologic element. With mounting evidence that Gd retention has clinical consequences, patients should be provided proper informed consent. Complications of renal insufficiency (ie, hyperkalemia, hyperphosphatemia, renal osteodystrophy, hyponatremia, anemia, immunosuppression, etc) follow a smooth, curvilinear slope as the true (not estimated) glomerular filtration declines; the worst iatrogenic complication from Gd—systemic fibrosis—is likely no different.
Patient Perspective
“Seems like it’s one thing after another. My family doctor said that once I had the gadolinium exposures, I have had problems ever since that I don’t recover from.” This includes chronic numbness from the rectum to the bilateral lower extremities and an indolent worsening kidney function; “I have already developed stage 3B chronic kidney disease.” Similar to many suffering with gadolinium retention, the patient was concerned about the long-term consequences. Gadolinium “is a toxic metal that is going through my body for 4 years. That has to be a problem. How come we don’t have that answer?” Clinician ignorance of Gd-induced complications and long-term retention is frustrating. “Not one of my doctors has taken gadolinium retention seriously. Where else are patients supposed to go?”
Conclusions
Health care professionals should be considering subclinical manifestations of nephrogenic systemic fibrosis or open to considering that intracellular neuronal retention of Gd may correlate with symptoms arising after MRI contrast exposures. The science concerning the mechanisms of how Gd exerts its pathologic effects is lagging behind the commercialization of enhancing Gd elimination (ie, chelation therapies) and other untested remedies. Practitioners need to acknowledge the unknown potential consequences of Gd and listen to patients who suspect chronic adverse effects.
1. Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28(2):154-162. doi:10.1097/MNH.0000000000000475
2. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?. Nephrol Dial Transplant. 2006;21(4):1104-1108. doi:10.1093/ndt/gfk062
3. Do C, Barnes JL, Tan C, Wagner B. Type of MRI contrast, tissue gadolinium, and fibrosis. Am J Physiol Renal Physiol. 2014;307(7):F844-F855. doi:10.1152/ajprenal.00379.2014
4. Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
5. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311(1):F1-F11. doi:10.1152/ajprenal.00166.2016
6. Drel VR, Tan C, Barnes JL, Gorin Y, Lee DY, Wagner B. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
7. Do C, Drel V, Tan C, Lee D, Wagner B. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143.e2. doi:10.1016/j.jid.2019.03.1145
8. Do C, Ford B, Lee DY, Tan C, Escobar P, Wagner B. Gadolinium-based contrast agents: stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
9. Hirano S, Suzuki KT. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect. 1996;104(suppl 1):85-95. doi:10.1289/ehp.96104s185
10. Alwasiyah D, Murphy C, Jannetto P, Hogg M, Beuhler MC. Urinary gadolinium levels after contrast-enhanced MRI in individuals with normal renal function: a pilot study. J Med Toxicol. 2019;15(2):121-127. doi:10.1007/s13181-018-0693-1
11. Williams S, Grimm H. gadolinium toxicity: shedding light on the effects of retained gadolinium from contrast MRI. Accessed April 11, 2022. https://gdtoxicity.files.wordpress.com/2018/12/gadolinium-clearance-times-for-135-contrast-mri-cases-final-v1-1.pdf
12. DeBevits JJ, Reshma M, Bageac D, et al. Gray matter nucleus hyperintensity after monthly triple-dose gadopentetate dimeglumine with long-term magnetic resonance imaging. Invest Radiol. 2020;55(10):629-635. doi:10.1097/RLI.0000000000000663
13. Gathings RM, Reddy R, Santa Cruz D, Brodell RT. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151(3):316-319. doi:10.1001/jamadermatol.2014.2660
14. Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65(6):1095-1106 e7. doi:10.1016/j.jaad.2010.08.041
15. Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. Am J Kidney Dis. 2005;46(4):754-759. doi:10.1053/j.ajkd.2005.06.024
16. Ortonne N, Lipsker D, Chantrel F, Boehm N, Grosshans E, Cribier B. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150(5):1050-1052. doi:10.1111/j.1365-2133.2004.05900.x
17. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-49. doi:10.1016/j.semarthrit.2005.08.002
18. Lewis KG, Lester BW, Pan TD, Robinson-Bostom L. Nephrogenic fibrosing dermopathyand calciphylaxis with pseudoxanthoma elasticum-like changes. J Cutan Pathol. 2006;33(10):695-700. doi:10.1111/j.1600-0560.2006.00490.x
19. Gibson SE, Farver CF, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch Pathol Lab Med. 2006;130(2):209-212. doi:10.5858/2006-130-209-MIINFD
20. Cassis TB, Jackson JM, Sonnier GB, Callen JP. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45(1):56-59. doi:10.1111/j.1365-4632.2005.02701.x
21. Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol. 2006;54(suppl 2):S31-S34. doi:10.1016/j.jaad.2005.04.024
22. Sanyal S, Marckmann P, Scherer S, Abraham JL. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis—an autopsy-based review. Nephrol Dial Transplant. 2011;26(11):3616-3626. doi:10.1093/ndt/gfr085
23. Kucher C, Xu X, Pasha T, Elenitsas R. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol. 2005;32(7):484-490. doi:10.1111/j.0303-6987.2005.00365.x
24. Goldstein KM, Lunyera J, Mohottige D, et al. Risk of Nephrogenic Systemic Fibrosis after Exposure to Newer Gadolinium Agents. Washington (DC): Department of Veterans Affairs (US); October 2019. https://www.ncbi.nlm.nih.gov/books/NBK559376/25. Lunyera J, Mohottige D, Alexopoulos AS, et al. Risk for nephrogenic systemic fibrosis after exposure to newer gadolinium agents: a systematic review. Ann Intern Med. 2020;173(2):110-119. doi:10.7326/M20-0299
26. Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH Oxidase 4 (Nox4) for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
27. Wagner B. The pathophysiology and retention of gadolinium. United States Food & Drug Administration Medical Imaging Drugs Advisory Committee. 2017:1-23. https://www.fda.gov/advisory-committees/medical-imaging-drugs-advisory-committee/2017-meeting-materials-medical-imaging-drugs-advisory-committee?msclkid=6b5764ccbaa611ec95e35dddf8db57af
28. Runge VM. Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the EMA’s pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest Radiol. 2017;52(6):317-323. doi:10.1097/RLI.0000000000000374
29. Wagner B. Scared to the marrow: pitfalls and pearls in renal imaging. Adv Chronic Kidney Dis. 2017;24(3):136-137. doi:10.1053/j.ackd.2017.03.008
30. US Food and Drug Administration. Transcript for the September 8, 2017 Meeting of the Medical Imaging Drugs Advisory Committee (MIDAC). September 8, 2017. Accessed April 11, 2022. https://www.fda.gov/media/108935/download
31. Abel M, Talbot RB. Gadolinium oxide inhalation by guinea pigs: a correlative functional and histopathologic study. J Pharmacol Exp Ther. 1967;157(1):207-213.
32. Haley TJ, Raymond K, Komesu N, Upham HC. Toxicological and pharmacological effects of gadolinium and samarium chlorides. Br J Pharmacol Chemother. 1961;17(3):526-532. doi:10.1111/j.1476-5381.1961.tb01139.x

33. Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E. Gadolinium chloride toxicity in the rat. Toxicol Pathol. 1997;25(3):245-255. doi:10.1177/019262339702500301
34. Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in humans: a family of disorders. AJR Am J Roentgenol. 2016;207(2):229-233. doi:10.2214/AJR.15.15842
35. Semelka RC, Ramalho M. Physicians with self-diagnosed gadolinium deposition disease: a case series. Radiol Bras. 2021;54(4):238-242. doi:10.1590/0100-3984.2020.0073
36. Layne KA, Wood DM, Dargan PI. Gadolinium-based contrast agents—what is the evidence for ‘gadolinium deposition disease’ and the use of chelation therapy? Clin Toxicol (Phila). 2020;58(3):151-160. doi:10.1080/15563650.2019.1681442
37. Nehra AK, McDonald RJ, Bluhm AM, et al. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: a prospective observational cohort study. Radiology. 2018;288(2):416-423. doi:10.1148/radiol.2018171105
38. US Food and Drug Administration. Medical Imaging Drugs Advisory Committee Meeting. Gadolinium retention after gadolinium based contrast magnetic resonance imaging in patients with normal renal function. Briefing document. 2017. Accessed April 12, 2022. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM572848.pdf
39. Calvo N, Jamil M, Feldman S, Shah A, Nauman F, Ferrara J. Neurotoxicity from intrathecal gadolinium administration: case presentation and brief review. Neurol Clin Pract. 2020;10(1):e7-e10. doi:10.1212/CPJ.0000000000000696
40. Bower DV, Richter JK, von Tengg-Kobligk H, Heverhagen JT, Runge VM. Gadolinium-based MRI contrast agents induce mitochondrial toxicity and cell death in human neurons, and toxicity increases with reduced kinetic stability of the agent. Invest Radiol. 2019;54(8):453-463. doi:10.1097/RLI.0000000000000567
41. McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
42. Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1(6):561-568. doi:10.34067/KID.0000272019
43. Di Gregorio E, Furlan C, Atlante S, Stefania R, Gianolio E, Aime S. Gadolinium retention in erythrocytes and leukocytes from human and murine blood upon treatment with gadolinium-based contrast agents for magnetic resonance imaging. Invest Radiol. 2020;55(1):30-37. doi:10.1097/RLI.0000000000000608
44. Maecker HT, Siebert JC, Rosenberg-Hasson Y, Koran LM, Ramalho M, Semelka RC. Acute chelation therapy-associated changes in urine gadolinium, self-reported flare severity, and serum cytokines in gadolinium deposition disease. Invest Radiol. 2021;56(6):374-384. doi:10.1097/RLI.0000000000000752
45. Maecker HT, Wang W, Rosenberg-Hasson Y, Semelka RC, Hickey J, Koran LM. An initial investigation of serum cytokine levels in patients with gadolinium retention. Radiol Bras. 2020;53(5):306-313. doi:10.1590/0100-3984.2019.0075
46. Birka M, Wentker KS, Lusmöller E, et al. Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem. 2015;87(6):3321-3328. doi:10.1021/ac504488k
Gadolinium (Gd)-based contrast agents are frequently used in health care for enhancing magnetic resonance image (MRI) signals at low concentrations. Contrary to popular opinion, this widely used heavy metal is not biologically inert. Once notable for its safety profile, there is mounting evidence for Gd deposition in various organ systems of the body, even in those with normal renal function. A large knowledge gap remains concerning the potential harms of Gd deposition and the factors determining its elimination from the body. However, the findings of deposited Gd throughout various organs and their intracellular compartments even years after the initial exposure have been established. Here, we describe a case of a Vietnam-era veteran whose presentation, clinical, and laboratory findings were consistent within the spectrum of Gd deposition disease.
Case Presentation
A Vietnam-era veteran aged > 70 years presented for evaluation of Gd-based contrast agent–induced chronic multisymptomatic illness His medical history was significant for chronic low back pain, chronic hypertension, type 2 diabetes mellitus, and hypogonadism. Surgical history was notable for back surgery (24 years prior), laminectomy (2 years prior), shoulder replacement (2 years prior), and an epidural complicated by a hematoma (1 year prior). His presenting concerns included a painful and pruritic rash that worsened with showering, pain originating at the right Achilles tendon with migration to the knee, and shoulder pain. His symptoms started shortly after receiving multiple exposures to Gd-based contrast agents to enhance MRIs during his clinical care (Omniscan 20 mL, Omniscan 20 mL, and Gadovist 10 mL, administered 578, 565, and 496 days prior to the clinic visit, respectively). New onset headaches coincided with the timeline of symptom onset, in addition to hoarseness and liberation of an “oily substance” from the skin. More than one year prior to this clinic visit, he was considered for having polymyalgia rheumatica given the ambiguity of symptoms. Functional status remained impaired despite treatment with prednisone and methotrexate.
The patient’s military service was in the mid-1960s. He was deployed to Japan and had no knowledge of an Agent Orange exposure. His tobacco history was distant, and he reported no tattoos, prior transfusions, or occupational metal exposure
Clinical Findings
The patient was afebrile, normotensive (146/88 mmHg), and normocardic. His weight was 100 kg. He was well nourished and in no acute distress. The thought process was attentive, and his affect pleasant. Ocular examination was notable for arcus senilus. The fundoscopic examination was limited on the left, but there was no neovascularization on the right. Jugular venous pulsation was normal at 8 cm. Right ventricular impulse was slightly hyperdynamic, the rhythm was regular, and there was no abnormal splitting of S2. A soft-grade I/VI crescendo/decrescendo murmur was auscultated along the apex. Radial pulses were 2/2. He was not in respiratory distress, with equally resonant fields bilaterally. Lung sounds were clear bilaterally. A papular, erythematous rash was present in a general distribution over the chest, with few telangiectasias and some varicosity along his left arm. The skin had normal elasticity, although the skin of the hands and legs was papyraceous.
Gd levels were measured in the blood and urine (Table 1). Gd was detectable in the skin (0.2 µg/g) nearly 400 days after the last exposure. Gd was still detectable in the patient’s blood and urine (0.2 ng/mL and 0.5 µg/24 h, respectively) more than 3 years after his last exposure.
Discussion
In the United States, there are 40.44 MRI units per million people and 40 million MRIs are conducted annually. From 30 to 50% of these are enhanced with Gd-based contrast agents. In the past 30 years, there have been > 450 million contrast-enhanced MRI procedures.1
Gd is a rare earth metal. Among commercially available elements Gd has exceptional properties for enhancing MRI signals at low concentrations.1 The nonphysiologic metal is detoxified by chelation with proprietary multidentate formulations that enhance (primarily renal) elimination while retaining the paramagnetic and chemical properties for imaging. Gd exposure was found to be associated to iatrogenic nephrogenic systemic fibrosis in 2006 and later confirmed via multiple systematic reviews.2 Gd is retained in every vital organ after exposure.3 Gd-based contrast agents stimulate bone marrow–derived fibrocytes in mediating fibrosis, and bone marrow develop a memory of prior contrast exposure (Figure 1).4-6 Systemic fibrosis is mediated by the monocyte chemoattractant protein 1/C-C chemokine receptor 2.6,7 Even in the setting of normal renal function, Gd-based contrast induces the formation of Gd-rich nanoparticles in the skin and kidney.7,8 Far from being inert, Gd-based contrast agents induce systemic metabolic changes such as hypertriglyceridemia, elevations in low-density lipoprotein cholesterol, insulin resistance, and the Warburg effect (glycolytic/energy switching) in the renal cortex concomitant with profound mitochondrial abnormalities.8
We have discovered that the rate of Gd-enhanced procedures has increased immensely within the Veterans Health Administration (VHA) system in a subset of patients with designated kidney disease (Table 2). Although a substantial number of procedures are dedicated to head and brain imaging within the VHA, the indications for Gd-enhanced diagnoses (eg, cardiac) are increasing (Figure 2).
Retention of Gd can be modeled as a function of time (t) by the half-lives of the fast, intermediate, and slow phases of elimination (Ta, Tb, and Tc, respectively):9
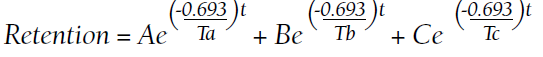
A, B, and C are the proportions (adding to 100%) that represent each of the compartments:
Numerous patients with normal renal function developed similar or novel symptoms that have been attributed to Gd concomitant with detectable urinary Gd years after exposure.11 Gd-based contrast agents are increasingly associated with cutaneous abnormalities even outside of nephrogenic systemic fibrosis. Gd-associated plaques develop in patients without kidney disease—these range from asymptomatic, pruritic, to burning.13 Histologic specimens reveal CD68 and factor XIIIa–positive spindle-shaped myeloid cells (the same mediators of iatrogenic systemic fibrosis) or CD34-positive cells. CD68 and factor XIIIa are distinctive for histologic specimens from patients with systemic fibrosis, and these markers have been detected in our preclinical models that demonstrated that bone marrow–derived cells are involved in mediating fibrosis.3,4,14-19 Similarly, CD34-positive cells have been historically associated with systemic fibrosis lesions.15,16,18-23 Plump osteocyte-appearing cells have also been noted (note that extraosseous metaplasia makes the histologic diagnosis of systemic fibrosis).14 Nephrogenic systemic fibrosis is an iatrogenic disease that can manifest years after exposure to Gd.5 Gd induces the recruitment of bone marrow–derived cells to the affected sites.4
The VA Health Service Research and Development Evidence Synthesis Program reviewed the safety of Gd-based contrast agents in patients with impaired kidney function.24,25 The group found only a single study of Gd and veterans. “Awareness and concern are growing about the long-term deposition of gadolinium in [the] brain and other tissues among patients with normal kidney function,” according to Lunyera and colleagues.25 The largest knowledge gap was that a comprehensive review “of all potential harms associated with gadolinium exposure” was not addressed. Furthermore, the group advised “caution in the use of [Gd-based contrast agents] in patients with severely impaired kidney function and acute kidney injury remains prudent, because the exact clinical factors contributing to [nephrogenic systemic fibrosis] risk in these subpopulations are still unknown.”25
Gd-based contrast agents—contrary to a widely held misconception—are not biologically inert.1 Gd-based contrast agents have a long history of association with acute renal injury. We have demonstrated that systemic treatment with MRI contrast agents leads to vacuolization of the proximal tubule and tubular injury.7,8 Kidney injury may be mediated by the generation of reactive oxygen species from NADPH oxidase 4 (Nox4).26
Gd retention, Gd-induced multisymptomatic illnesses, Gd-associated plaques, Gd-induced neurotoxicity, and nephrogenic systemic fibrosis are part of a continuum (with Gd as the common thread)—a theme of the September 8, 2017, US Food and Drug Administration (FDA) Medical Imaging Drugs Advisory Committee meeting.27 Patients, patient advocacy groups, and regulating agencies are concerned about long-term retention of a nonphysiologic rare earth element such as Gd.28-30 A patient advocacy group, The Lighthouse Project, collected information from patients linking the last date of Gd-based contrast agent exposure and urinary Gd.11 Data from their report suggest that the rate constants (valuable for the elimination equation above) are obtainable from 24-hour urine collections. Conceptually, Gd-induced diseases may represent a continuum that results from the retention of a nonphysiologic, toxic heavy rare earth metal.
As a heavy metal, Gd is not a natural physiologic trace element. Similar to numerous nonphysiologic metals, Gd is toxic. Inhaled Gd oxide (Gd2O3) dust leads to a number of time-dependent pathologies. Animal lung studies demonstrate reduced elasticity, enlarged cells, thickened lung walls, and recruitment of immune cells.31 Symptoms of acute IV Gd toxicity include decreased respiration, lethargy, abdominal cramps, and diarrhea.32 Pharmacologically, Gd concentrates in the liver and kidney and accumulates in the bone.32 Animals demonstrate intestinal depression and low blood pressure in response to Gd and, with higher doses, cardiovascular collapse.32 IV Gd chloride leads to metal deposition in the small blood vessels diffusely throughout the body, particularly in the lung and kidney and the metal is absorbed by the scavenging white blood cells.33 Gd chloride induces severe damage to the liver, spleen, and the digestive tract.33 Furthermore, this form of the toxicant metal markedly impacted functions associated with bleeding and clotting, ie, decreased platelet numbers and an increase in the laboratory-measured coagulation parameters.33 Semelka and colleagues have characterized chronic symptoms attributed to Gd-based contrast agents (not limited to chronic pain, headache, bone pain, skin thickening, and clouded mentation).34,35 Because Gd-induced conditions are underrecognized and ill-defined, disinherited patients often resort to untested (and potentially dangerous) chelation therapies.36
This patient presented with numerous symptoms that arose after Gd exposure. It is well established that Gd-based contrast agents (of any class) are retained in multiple organs (including the brain), for months to years. Gd-based contrast agents enter the cerebrospinal fluid within minutes of IV administration.37 Gd was found in the cerebrospinal fluid 9 months after administration in a case presented to the FDA Medical Imaging Drugs Advisory Committee.38 We know from intentional and accidental intrathecal administrations that Gd-based contrast agents are neurotoxic.39 Runge and colleagues demonstrated that Gd-based contrast agents exert mitochondrial toxicity in cultured neurons in vitro.40 McDonald and his team found Gd-rich nanoparticles within the brain neurons (cytoplasm and nuclei) from patients exposed to MRI contrast in the normal course of care.41 These nanoparticles are similar to what we have found in rodent models of Gd-induced disease.7,8,42
Prolonged elimination of Gd after MRI contrast administration (months to years) may be universal.10 Gd compartmentalizes into leukocytes and erythrocytes and into the cerebrospinal fluid within minutes.37,43 Patients with multisymptomatic illnesses attributed to Gd (Gd deposition disease) have perturbations in cytokine levels, many inflammatory.44,45 The results are concerning: Gd is retained intracellularly in vital organs, including brain neurons. It is inarguable that Gd is an alien, nonphysiologic element. With mounting evidence that Gd retention has clinical consequences, patients should be provided proper informed consent. Complications of renal insufficiency (ie, hyperkalemia, hyperphosphatemia, renal osteodystrophy, hyponatremia, anemia, immunosuppression, etc) follow a smooth, curvilinear slope as the true (not estimated) glomerular filtration declines; the worst iatrogenic complication from Gd—systemic fibrosis—is likely no different.
Patient Perspective
“Seems like it’s one thing after another. My family doctor said that once I had the gadolinium exposures, I have had problems ever since that I don’t recover from.” This includes chronic numbness from the rectum to the bilateral lower extremities and an indolent worsening kidney function; “I have already developed stage 3B chronic kidney disease.” Similar to many suffering with gadolinium retention, the patient was concerned about the long-term consequences. Gadolinium “is a toxic metal that is going through my body for 4 years. That has to be a problem. How come we don’t have that answer?” Clinician ignorance of Gd-induced complications and long-term retention is frustrating. “Not one of my doctors has taken gadolinium retention seriously. Where else are patients supposed to go?”
Conclusions
Health care professionals should be considering subclinical manifestations of nephrogenic systemic fibrosis or open to considering that intracellular neuronal retention of Gd may correlate with symptoms arising after MRI contrast exposures. The science concerning the mechanisms of how Gd exerts its pathologic effects is lagging behind the commercialization of enhancing Gd elimination (ie, chelation therapies) and other untested remedies. Practitioners need to acknowledge the unknown potential consequences of Gd and listen to patients who suspect chronic adverse effects.
Gadolinium (Gd)-based contrast agents are frequently used in health care for enhancing magnetic resonance image (MRI) signals at low concentrations. Contrary to popular opinion, this widely used heavy metal is not biologically inert. Once notable for its safety profile, there is mounting evidence for Gd deposition in various organ systems of the body, even in those with normal renal function. A large knowledge gap remains concerning the potential harms of Gd deposition and the factors determining its elimination from the body. However, the findings of deposited Gd throughout various organs and their intracellular compartments even years after the initial exposure have been established. Here, we describe a case of a Vietnam-era veteran whose presentation, clinical, and laboratory findings were consistent within the spectrum of Gd deposition disease.
Case Presentation
A Vietnam-era veteran aged > 70 years presented for evaluation of Gd-based contrast agent–induced chronic multisymptomatic illness His medical history was significant for chronic low back pain, chronic hypertension, type 2 diabetes mellitus, and hypogonadism. Surgical history was notable for back surgery (24 years prior), laminectomy (2 years prior), shoulder replacement (2 years prior), and an epidural complicated by a hematoma (1 year prior). His presenting concerns included a painful and pruritic rash that worsened with showering, pain originating at the right Achilles tendon with migration to the knee, and shoulder pain. His symptoms started shortly after receiving multiple exposures to Gd-based contrast agents to enhance MRIs during his clinical care (Omniscan 20 mL, Omniscan 20 mL, and Gadovist 10 mL, administered 578, 565, and 496 days prior to the clinic visit, respectively). New onset headaches coincided with the timeline of symptom onset, in addition to hoarseness and liberation of an “oily substance” from the skin. More than one year prior to this clinic visit, he was considered for having polymyalgia rheumatica given the ambiguity of symptoms. Functional status remained impaired despite treatment with prednisone and methotrexate.
The patient’s military service was in the mid-1960s. He was deployed to Japan and had no knowledge of an Agent Orange exposure. His tobacco history was distant, and he reported no tattoos, prior transfusions, or occupational metal exposure
Clinical Findings
The patient was afebrile, normotensive (146/88 mmHg), and normocardic. His weight was 100 kg. He was well nourished and in no acute distress. The thought process was attentive, and his affect pleasant. Ocular examination was notable for arcus senilus. The fundoscopic examination was limited on the left, but there was no neovascularization on the right. Jugular venous pulsation was normal at 8 cm. Right ventricular impulse was slightly hyperdynamic, the rhythm was regular, and there was no abnormal splitting of S2. A soft-grade I/VI crescendo/decrescendo murmur was auscultated along the apex. Radial pulses were 2/2. He was not in respiratory distress, with equally resonant fields bilaterally. Lung sounds were clear bilaterally. A papular, erythematous rash was present in a general distribution over the chest, with few telangiectasias and some varicosity along his left arm. The skin had normal elasticity, although the skin of the hands and legs was papyraceous.
Gd levels were measured in the blood and urine (Table 1). Gd was detectable in the skin (0.2 µg/g) nearly 400 days after the last exposure. Gd was still detectable in the patient’s blood and urine (0.2 ng/mL and 0.5 µg/24 h, respectively) more than 3 years after his last exposure.
Discussion
In the United States, there are 40.44 MRI units per million people and 40 million MRIs are conducted annually. From 30 to 50% of these are enhanced with Gd-based contrast agents. In the past 30 years, there have been > 450 million contrast-enhanced MRI procedures.1
Gd is a rare earth metal. Among commercially available elements Gd has exceptional properties for enhancing MRI signals at low concentrations.1 The nonphysiologic metal is detoxified by chelation with proprietary multidentate formulations that enhance (primarily renal) elimination while retaining the paramagnetic and chemical properties for imaging. Gd exposure was found to be associated to iatrogenic nephrogenic systemic fibrosis in 2006 and later confirmed via multiple systematic reviews.2 Gd is retained in every vital organ after exposure.3 Gd-based contrast agents stimulate bone marrow–derived fibrocytes in mediating fibrosis, and bone marrow develop a memory of prior contrast exposure (Figure 1).4-6 Systemic fibrosis is mediated by the monocyte chemoattractant protein 1/C-C chemokine receptor 2.6,7 Even in the setting of normal renal function, Gd-based contrast induces the formation of Gd-rich nanoparticles in the skin and kidney.7,8 Far from being inert, Gd-based contrast agents induce systemic metabolic changes such as hypertriglyceridemia, elevations in low-density lipoprotein cholesterol, insulin resistance, and the Warburg effect (glycolytic/energy switching) in the renal cortex concomitant with profound mitochondrial abnormalities.8
We have discovered that the rate of Gd-enhanced procedures has increased immensely within the Veterans Health Administration (VHA) system in a subset of patients with designated kidney disease (Table 2). Although a substantial number of procedures are dedicated to head and brain imaging within the VHA, the indications for Gd-enhanced diagnoses (eg, cardiac) are increasing (Figure 2).
Retention of Gd can be modeled as a function of time (t) by the half-lives of the fast, intermediate, and slow phases of elimination (Ta, Tb, and Tc, respectively):9
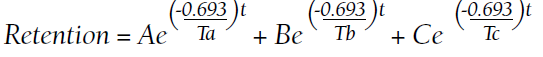
A, B, and C are the proportions (adding to 100%) that represent each of the compartments:
Numerous patients with normal renal function developed similar or novel symptoms that have been attributed to Gd concomitant with detectable urinary Gd years after exposure.11 Gd-based contrast agents are increasingly associated with cutaneous abnormalities even outside of nephrogenic systemic fibrosis. Gd-associated plaques develop in patients without kidney disease—these range from asymptomatic, pruritic, to burning.13 Histologic specimens reveal CD68 and factor XIIIa–positive spindle-shaped myeloid cells (the same mediators of iatrogenic systemic fibrosis) or CD34-positive cells. CD68 and factor XIIIa are distinctive for histologic specimens from patients with systemic fibrosis, and these markers have been detected in our preclinical models that demonstrated that bone marrow–derived cells are involved in mediating fibrosis.3,4,14-19 Similarly, CD34-positive cells have been historically associated with systemic fibrosis lesions.15,16,18-23 Plump osteocyte-appearing cells have also been noted (note that extraosseous metaplasia makes the histologic diagnosis of systemic fibrosis).14 Nephrogenic systemic fibrosis is an iatrogenic disease that can manifest years after exposure to Gd.5 Gd induces the recruitment of bone marrow–derived cells to the affected sites.4
The VA Health Service Research and Development Evidence Synthesis Program reviewed the safety of Gd-based contrast agents in patients with impaired kidney function.24,25 The group found only a single study of Gd and veterans. “Awareness and concern are growing about the long-term deposition of gadolinium in [the] brain and other tissues among patients with normal kidney function,” according to Lunyera and colleagues.25 The largest knowledge gap was that a comprehensive review “of all potential harms associated with gadolinium exposure” was not addressed. Furthermore, the group advised “caution in the use of [Gd-based contrast agents] in patients with severely impaired kidney function and acute kidney injury remains prudent, because the exact clinical factors contributing to [nephrogenic systemic fibrosis] risk in these subpopulations are still unknown.”25
Gd-based contrast agents—contrary to a widely held misconception—are not biologically inert.1 Gd-based contrast agents have a long history of association with acute renal injury. We have demonstrated that systemic treatment with MRI contrast agents leads to vacuolization of the proximal tubule and tubular injury.7,8 Kidney injury may be mediated by the generation of reactive oxygen species from NADPH oxidase 4 (Nox4).26
Gd retention, Gd-induced multisymptomatic illnesses, Gd-associated plaques, Gd-induced neurotoxicity, and nephrogenic systemic fibrosis are part of a continuum (with Gd as the common thread)—a theme of the September 8, 2017, US Food and Drug Administration (FDA) Medical Imaging Drugs Advisory Committee meeting.27 Patients, patient advocacy groups, and regulating agencies are concerned about long-term retention of a nonphysiologic rare earth element such as Gd.28-30 A patient advocacy group, The Lighthouse Project, collected information from patients linking the last date of Gd-based contrast agent exposure and urinary Gd.11 Data from their report suggest that the rate constants (valuable for the elimination equation above) are obtainable from 24-hour urine collections. Conceptually, Gd-induced diseases may represent a continuum that results from the retention of a nonphysiologic, toxic heavy rare earth metal.
As a heavy metal, Gd is not a natural physiologic trace element. Similar to numerous nonphysiologic metals, Gd is toxic. Inhaled Gd oxide (Gd2O3) dust leads to a number of time-dependent pathologies. Animal lung studies demonstrate reduced elasticity, enlarged cells, thickened lung walls, and recruitment of immune cells.31 Symptoms of acute IV Gd toxicity include decreased respiration, lethargy, abdominal cramps, and diarrhea.32 Pharmacologically, Gd concentrates in the liver and kidney and accumulates in the bone.32 Animals demonstrate intestinal depression and low blood pressure in response to Gd and, with higher doses, cardiovascular collapse.32 IV Gd chloride leads to metal deposition in the small blood vessels diffusely throughout the body, particularly in the lung and kidney and the metal is absorbed by the scavenging white blood cells.33 Gd chloride induces severe damage to the liver, spleen, and the digestive tract.33 Furthermore, this form of the toxicant metal markedly impacted functions associated with bleeding and clotting, ie, decreased platelet numbers and an increase in the laboratory-measured coagulation parameters.33 Semelka and colleagues have characterized chronic symptoms attributed to Gd-based contrast agents (not limited to chronic pain, headache, bone pain, skin thickening, and clouded mentation).34,35 Because Gd-induced conditions are underrecognized and ill-defined, disinherited patients often resort to untested (and potentially dangerous) chelation therapies.36
This patient presented with numerous symptoms that arose after Gd exposure. It is well established that Gd-based contrast agents (of any class) are retained in multiple organs (including the brain), for months to years. Gd-based contrast agents enter the cerebrospinal fluid within minutes of IV administration.37 Gd was found in the cerebrospinal fluid 9 months after administration in a case presented to the FDA Medical Imaging Drugs Advisory Committee.38 We know from intentional and accidental intrathecal administrations that Gd-based contrast agents are neurotoxic.39 Runge and colleagues demonstrated that Gd-based contrast agents exert mitochondrial toxicity in cultured neurons in vitro.40 McDonald and his team found Gd-rich nanoparticles within the brain neurons (cytoplasm and nuclei) from patients exposed to MRI contrast in the normal course of care.41 These nanoparticles are similar to what we have found in rodent models of Gd-induced disease.7,8,42
Prolonged elimination of Gd after MRI contrast administration (months to years) may be universal.10 Gd compartmentalizes into leukocytes and erythrocytes and into the cerebrospinal fluid within minutes.37,43 Patients with multisymptomatic illnesses attributed to Gd (Gd deposition disease) have perturbations in cytokine levels, many inflammatory.44,45 The results are concerning: Gd is retained intracellularly in vital organs, including brain neurons. It is inarguable that Gd is an alien, nonphysiologic element. With mounting evidence that Gd retention has clinical consequences, patients should be provided proper informed consent. Complications of renal insufficiency (ie, hyperkalemia, hyperphosphatemia, renal osteodystrophy, hyponatremia, anemia, immunosuppression, etc) follow a smooth, curvilinear slope as the true (not estimated) glomerular filtration declines; the worst iatrogenic complication from Gd—systemic fibrosis—is likely no different.
Patient Perspective
“Seems like it’s one thing after another. My family doctor said that once I had the gadolinium exposures, I have had problems ever since that I don’t recover from.” This includes chronic numbness from the rectum to the bilateral lower extremities and an indolent worsening kidney function; “I have already developed stage 3B chronic kidney disease.” Similar to many suffering with gadolinium retention, the patient was concerned about the long-term consequences. Gadolinium “is a toxic metal that is going through my body for 4 years. That has to be a problem. How come we don’t have that answer?” Clinician ignorance of Gd-induced complications and long-term retention is frustrating. “Not one of my doctors has taken gadolinium retention seriously. Where else are patients supposed to go?”
Conclusions
Health care professionals should be considering subclinical manifestations of nephrogenic systemic fibrosis or open to considering that intracellular neuronal retention of Gd may correlate with symptoms arising after MRI contrast exposures. The science concerning the mechanisms of how Gd exerts its pathologic effects is lagging behind the commercialization of enhancing Gd elimination (ie, chelation therapies) and other untested remedies. Practitioners need to acknowledge the unknown potential consequences of Gd and listen to patients who suspect chronic adverse effects.
1. Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28(2):154-162. doi:10.1097/MNH.0000000000000475
2. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?. Nephrol Dial Transplant. 2006;21(4):1104-1108. doi:10.1093/ndt/gfk062
3. Do C, Barnes JL, Tan C, Wagner B. Type of MRI contrast, tissue gadolinium, and fibrosis. Am J Physiol Renal Physiol. 2014;307(7):F844-F855. doi:10.1152/ajprenal.00379.2014
4. Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
5. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311(1):F1-F11. doi:10.1152/ajprenal.00166.2016
6. Drel VR, Tan C, Barnes JL, Gorin Y, Lee DY, Wagner B. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
7. Do C, Drel V, Tan C, Lee D, Wagner B. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143.e2. doi:10.1016/j.jid.2019.03.1145
8. Do C, Ford B, Lee DY, Tan C, Escobar P, Wagner B. Gadolinium-based contrast agents: stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
9. Hirano S, Suzuki KT. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect. 1996;104(suppl 1):85-95. doi:10.1289/ehp.96104s185
10. Alwasiyah D, Murphy C, Jannetto P, Hogg M, Beuhler MC. Urinary gadolinium levels after contrast-enhanced MRI in individuals with normal renal function: a pilot study. J Med Toxicol. 2019;15(2):121-127. doi:10.1007/s13181-018-0693-1
11. Williams S, Grimm H. gadolinium toxicity: shedding light on the effects of retained gadolinium from contrast MRI. Accessed April 11, 2022. https://gdtoxicity.files.wordpress.com/2018/12/gadolinium-clearance-times-for-135-contrast-mri-cases-final-v1-1.pdf
12. DeBevits JJ, Reshma M, Bageac D, et al. Gray matter nucleus hyperintensity after monthly triple-dose gadopentetate dimeglumine with long-term magnetic resonance imaging. Invest Radiol. 2020;55(10):629-635. doi:10.1097/RLI.0000000000000663
13. Gathings RM, Reddy R, Santa Cruz D, Brodell RT. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151(3):316-319. doi:10.1001/jamadermatol.2014.2660
14. Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65(6):1095-1106 e7. doi:10.1016/j.jaad.2010.08.041
15. Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. Am J Kidney Dis. 2005;46(4):754-759. doi:10.1053/j.ajkd.2005.06.024
16. Ortonne N, Lipsker D, Chantrel F, Boehm N, Grosshans E, Cribier B. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150(5):1050-1052. doi:10.1111/j.1365-2133.2004.05900.x
17. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-49. doi:10.1016/j.semarthrit.2005.08.002
18. Lewis KG, Lester BW, Pan TD, Robinson-Bostom L. Nephrogenic fibrosing dermopathyand calciphylaxis with pseudoxanthoma elasticum-like changes. J Cutan Pathol. 2006;33(10):695-700. doi:10.1111/j.1600-0560.2006.00490.x
19. Gibson SE, Farver CF, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch Pathol Lab Med. 2006;130(2):209-212. doi:10.5858/2006-130-209-MIINFD
20. Cassis TB, Jackson JM, Sonnier GB, Callen JP. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45(1):56-59. doi:10.1111/j.1365-4632.2005.02701.x
21. Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol. 2006;54(suppl 2):S31-S34. doi:10.1016/j.jaad.2005.04.024
22. Sanyal S, Marckmann P, Scherer S, Abraham JL. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis—an autopsy-based review. Nephrol Dial Transplant. 2011;26(11):3616-3626. doi:10.1093/ndt/gfr085
23. Kucher C, Xu X, Pasha T, Elenitsas R. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol. 2005;32(7):484-490. doi:10.1111/j.0303-6987.2005.00365.x
24. Goldstein KM, Lunyera J, Mohottige D, et al. Risk of Nephrogenic Systemic Fibrosis after Exposure to Newer Gadolinium Agents. Washington (DC): Department of Veterans Affairs (US); October 2019. https://www.ncbi.nlm.nih.gov/books/NBK559376/25. Lunyera J, Mohottige D, Alexopoulos AS, et al. Risk for nephrogenic systemic fibrosis after exposure to newer gadolinium agents: a systematic review. Ann Intern Med. 2020;173(2):110-119. doi:10.7326/M20-0299
26. Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH Oxidase 4 (Nox4) for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
27. Wagner B. The pathophysiology and retention of gadolinium. United States Food & Drug Administration Medical Imaging Drugs Advisory Committee. 2017:1-23. https://www.fda.gov/advisory-committees/medical-imaging-drugs-advisory-committee/2017-meeting-materials-medical-imaging-drugs-advisory-committee?msclkid=6b5764ccbaa611ec95e35dddf8db57af
28. Runge VM. Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the EMA’s pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest Radiol. 2017;52(6):317-323. doi:10.1097/RLI.0000000000000374
29. Wagner B. Scared to the marrow: pitfalls and pearls in renal imaging. Adv Chronic Kidney Dis. 2017;24(3):136-137. doi:10.1053/j.ackd.2017.03.008
30. US Food and Drug Administration. Transcript for the September 8, 2017 Meeting of the Medical Imaging Drugs Advisory Committee (MIDAC). September 8, 2017. Accessed April 11, 2022. https://www.fda.gov/media/108935/download
31. Abel M, Talbot RB. Gadolinium oxide inhalation by guinea pigs: a correlative functional and histopathologic study. J Pharmacol Exp Ther. 1967;157(1):207-213.
32. Haley TJ, Raymond K, Komesu N, Upham HC. Toxicological and pharmacological effects of gadolinium and samarium chlorides. Br J Pharmacol Chemother. 1961;17(3):526-532. doi:10.1111/j.1476-5381.1961.tb01139.x

33. Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E. Gadolinium chloride toxicity in the rat. Toxicol Pathol. 1997;25(3):245-255. doi:10.1177/019262339702500301
34. Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in humans: a family of disorders. AJR Am J Roentgenol. 2016;207(2):229-233. doi:10.2214/AJR.15.15842
35. Semelka RC, Ramalho M. Physicians with self-diagnosed gadolinium deposition disease: a case series. Radiol Bras. 2021;54(4):238-242. doi:10.1590/0100-3984.2020.0073
36. Layne KA, Wood DM, Dargan PI. Gadolinium-based contrast agents—what is the evidence for ‘gadolinium deposition disease’ and the use of chelation therapy? Clin Toxicol (Phila). 2020;58(3):151-160. doi:10.1080/15563650.2019.1681442
37. Nehra AK, McDonald RJ, Bluhm AM, et al. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: a prospective observational cohort study. Radiology. 2018;288(2):416-423. doi:10.1148/radiol.2018171105
38. US Food and Drug Administration. Medical Imaging Drugs Advisory Committee Meeting. Gadolinium retention after gadolinium based contrast magnetic resonance imaging in patients with normal renal function. Briefing document. 2017. Accessed April 12, 2022. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM572848.pdf
39. Calvo N, Jamil M, Feldman S, Shah A, Nauman F, Ferrara J. Neurotoxicity from intrathecal gadolinium administration: case presentation and brief review. Neurol Clin Pract. 2020;10(1):e7-e10. doi:10.1212/CPJ.0000000000000696
40. Bower DV, Richter JK, von Tengg-Kobligk H, Heverhagen JT, Runge VM. Gadolinium-based MRI contrast agents induce mitochondrial toxicity and cell death in human neurons, and toxicity increases with reduced kinetic stability of the agent. Invest Radiol. 2019;54(8):453-463. doi:10.1097/RLI.0000000000000567
41. McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
42. Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1(6):561-568. doi:10.34067/KID.0000272019
43. Di Gregorio E, Furlan C, Atlante S, Stefania R, Gianolio E, Aime S. Gadolinium retention in erythrocytes and leukocytes from human and murine blood upon treatment with gadolinium-based contrast agents for magnetic resonance imaging. Invest Radiol. 2020;55(1):30-37. doi:10.1097/RLI.0000000000000608
44. Maecker HT, Siebert JC, Rosenberg-Hasson Y, Koran LM, Ramalho M, Semelka RC. Acute chelation therapy-associated changes in urine gadolinium, self-reported flare severity, and serum cytokines in gadolinium deposition disease. Invest Radiol. 2021;56(6):374-384. doi:10.1097/RLI.0000000000000752
45. Maecker HT, Wang W, Rosenberg-Hasson Y, Semelka RC, Hickey J, Koran LM. An initial investigation of serum cytokine levels in patients with gadolinium retention. Radiol Bras. 2020;53(5):306-313. doi:10.1590/0100-3984.2019.0075
46. Birka M, Wentker KS, Lusmöller E, et al. Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem. 2015;87(6):3321-3328. doi:10.1021/ac504488k
1. Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28(2):154-162. doi:10.1097/MNH.0000000000000475
2. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?. Nephrol Dial Transplant. 2006;21(4):1104-1108. doi:10.1093/ndt/gfk062
3. Do C, Barnes JL, Tan C, Wagner B. Type of MRI contrast, tissue gadolinium, and fibrosis. Am J Physiol Renal Physiol. 2014;307(7):F844-F855. doi:10.1152/ajprenal.00379.2014
4. Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
5. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311(1):F1-F11. doi:10.1152/ajprenal.00166.2016
6. Drel VR, Tan C, Barnes JL, Gorin Y, Lee DY, Wagner B. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
7. Do C, Drel V, Tan C, Lee D, Wagner B. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143.e2. doi:10.1016/j.jid.2019.03.1145
8. Do C, Ford B, Lee DY, Tan C, Escobar P, Wagner B. Gadolinium-based contrast agents: stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
9. Hirano S, Suzuki KT. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect. 1996;104(suppl 1):85-95. doi:10.1289/ehp.96104s185
10. Alwasiyah D, Murphy C, Jannetto P, Hogg M, Beuhler MC. Urinary gadolinium levels after contrast-enhanced MRI in individuals with normal renal function: a pilot study. J Med Toxicol. 2019;15(2):121-127. doi:10.1007/s13181-018-0693-1
11. Williams S, Grimm H. gadolinium toxicity: shedding light on the effects of retained gadolinium from contrast MRI. Accessed April 11, 2022. https://gdtoxicity.files.wordpress.com/2018/12/gadolinium-clearance-times-for-135-contrast-mri-cases-final-v1-1.pdf
12. DeBevits JJ, Reshma M, Bageac D, et al. Gray matter nucleus hyperintensity after monthly triple-dose gadopentetate dimeglumine with long-term magnetic resonance imaging. Invest Radiol. 2020;55(10):629-635. doi:10.1097/RLI.0000000000000663
13. Gathings RM, Reddy R, Santa Cruz D, Brodell RT. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151(3):316-319. doi:10.1001/jamadermatol.2014.2660
14. Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65(6):1095-1106 e7. doi:10.1016/j.jaad.2010.08.041
15. Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. Am J Kidney Dis. 2005;46(4):754-759. doi:10.1053/j.ajkd.2005.06.024
16. Ortonne N, Lipsker D, Chantrel F, Boehm N, Grosshans E, Cribier B. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150(5):1050-1052. doi:10.1111/j.1365-2133.2004.05900.x
17. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-49. doi:10.1016/j.semarthrit.2005.08.002
18. Lewis KG, Lester BW, Pan TD, Robinson-Bostom L. Nephrogenic fibrosing dermopathyand calciphylaxis with pseudoxanthoma elasticum-like changes. J Cutan Pathol. 2006;33(10):695-700. doi:10.1111/j.1600-0560.2006.00490.x
19. Gibson SE, Farver CF, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch Pathol Lab Med. 2006;130(2):209-212. doi:10.5858/2006-130-209-MIINFD
20. Cassis TB, Jackson JM, Sonnier GB, Callen JP. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45(1):56-59. doi:10.1111/j.1365-4632.2005.02701.x
21. Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol. 2006;54(suppl 2):S31-S34. doi:10.1016/j.jaad.2005.04.024
22. Sanyal S, Marckmann P, Scherer S, Abraham JL. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis—an autopsy-based review. Nephrol Dial Transplant. 2011;26(11):3616-3626. doi:10.1093/ndt/gfr085
23. Kucher C, Xu X, Pasha T, Elenitsas R. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol. 2005;32(7):484-490. doi:10.1111/j.0303-6987.2005.00365.x
24. Goldstein KM, Lunyera J, Mohottige D, et al. Risk of Nephrogenic Systemic Fibrosis after Exposure to Newer Gadolinium Agents. Washington (DC): Department of Veterans Affairs (US); October 2019. https://www.ncbi.nlm.nih.gov/books/NBK559376/25. Lunyera J, Mohottige D, Alexopoulos AS, et al. Risk for nephrogenic systemic fibrosis after exposure to newer gadolinium agents: a systematic review. Ann Intern Med. 2020;173(2):110-119. doi:10.7326/M20-0299
26. Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH Oxidase 4 (Nox4) for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
27. Wagner B. The pathophysiology and retention of gadolinium. United States Food & Drug Administration Medical Imaging Drugs Advisory Committee. 2017:1-23. https://www.fda.gov/advisory-committees/medical-imaging-drugs-advisory-committee/2017-meeting-materials-medical-imaging-drugs-advisory-committee?msclkid=6b5764ccbaa611ec95e35dddf8db57af
28. Runge VM. Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the EMA’s pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest Radiol. 2017;52(6):317-323. doi:10.1097/RLI.0000000000000374
29. Wagner B. Scared to the marrow: pitfalls and pearls in renal imaging. Adv Chronic Kidney Dis. 2017;24(3):136-137. doi:10.1053/j.ackd.2017.03.008
30. US Food and Drug Administration. Transcript for the September 8, 2017 Meeting of the Medical Imaging Drugs Advisory Committee (MIDAC). September 8, 2017. Accessed April 11, 2022. https://www.fda.gov/media/108935/download
31. Abel M, Talbot RB. Gadolinium oxide inhalation by guinea pigs: a correlative functional and histopathologic study. J Pharmacol Exp Ther. 1967;157(1):207-213.
32. Haley TJ, Raymond K, Komesu N, Upham HC. Toxicological and pharmacological effects of gadolinium and samarium chlorides. Br J Pharmacol Chemother. 1961;17(3):526-532. doi:10.1111/j.1476-5381.1961.tb01139.x

33. Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E. Gadolinium chloride toxicity in the rat. Toxicol Pathol. 1997;25(3):245-255. doi:10.1177/019262339702500301
34. Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in humans: a family of disorders. AJR Am J Roentgenol. 2016;207(2):229-233. doi:10.2214/AJR.15.15842
35. Semelka RC, Ramalho M. Physicians with self-diagnosed gadolinium deposition disease: a case series. Radiol Bras. 2021;54(4):238-242. doi:10.1590/0100-3984.2020.0073
36. Layne KA, Wood DM, Dargan PI. Gadolinium-based contrast agents—what is the evidence for ‘gadolinium deposition disease’ and the use of chelation therapy? Clin Toxicol (Phila). 2020;58(3):151-160. doi:10.1080/15563650.2019.1681442
37. Nehra AK, McDonald RJ, Bluhm AM, et al. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: a prospective observational cohort study. Radiology. 2018;288(2):416-423. doi:10.1148/radiol.2018171105
38. US Food and Drug Administration. Medical Imaging Drugs Advisory Committee Meeting. Gadolinium retention after gadolinium based contrast magnetic resonance imaging in patients with normal renal function. Briefing document. 2017. Accessed April 12, 2022. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM572848.pdf
39. Calvo N, Jamil M, Feldman S, Shah A, Nauman F, Ferrara J. Neurotoxicity from intrathecal gadolinium administration: case presentation and brief review. Neurol Clin Pract. 2020;10(1):e7-e10. doi:10.1212/CPJ.0000000000000696
40. Bower DV, Richter JK, von Tengg-Kobligk H, Heverhagen JT, Runge VM. Gadolinium-based MRI contrast agents induce mitochondrial toxicity and cell death in human neurons, and toxicity increases with reduced kinetic stability of the agent. Invest Radiol. 2019;54(8):453-463. doi:10.1097/RLI.0000000000000567
41. McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
42. Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1(6):561-568. doi:10.34067/KID.0000272019
43. Di Gregorio E, Furlan C, Atlante S, Stefania R, Gianolio E, Aime S. Gadolinium retention in erythrocytes and leukocytes from human and murine blood upon treatment with gadolinium-based contrast agents for magnetic resonance imaging. Invest Radiol. 2020;55(1):30-37. doi:10.1097/RLI.0000000000000608
44. Maecker HT, Siebert JC, Rosenberg-Hasson Y, Koran LM, Ramalho M, Semelka RC. Acute chelation therapy-associated changes in urine gadolinium, self-reported flare severity, and serum cytokines in gadolinium deposition disease. Invest Radiol. 2021;56(6):374-384. doi:10.1097/RLI.0000000000000752
45. Maecker HT, Wang W, Rosenberg-Hasson Y, Semelka RC, Hickey J, Koran LM. An initial investigation of serum cytokine levels in patients with gadolinium retention. Radiol Bras. 2020;53(5):306-313. doi:10.1590/0100-3984.2019.0075
46. Birka M, Wentker KS, Lusmöller E, et al. Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem. 2015;87(6):3321-3328. doi:10.1021/ac504488k