User login
Depression carries a wide differential diagnosis. Practitioners sometimes think polarity is the fundamental distinction when they conceptualize depression as a clinical entity; in fact, many nosologic frameworks have been described for defining and subtyping clinically meaningful forms of depression, and each waxed and waned in popularity.
Kraepelin, writing in the early 20th century, linked manic-depressive illness with “the greater part of the morbid states termed melancholia,”1 but many features other than polarity remain important components of depression, and those features often carry implications for how individual patients respond to treatment.
In this 2-part article [April and May 2014 issues], I summarize information about clinically distinct subtypes of depression, as they are recognized within diagnostic systems or as descriptors of treatment outcomes for particular subgroups of patients. My focus is on practical considerations for assessing and managing depression. Because many forms of the disorder respond inadequately to initial antidepressant treatment, optimal “next-step” pharmacotherapy, after nonresponse or partial response, often hinges on clinical subtyping.
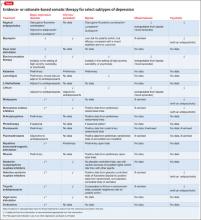
The first part of this article examines major depressive disorder (MDD), minor depression, chronic depression, depression in bipolar disorder, depression that is severe or mild, and psychotic depression. Treatments for these subtypes for which there is evidence, or a clinical rationale, are given in the Table.
The subtypes of depression that I’ll discuss in the second part of the article are listed on page 47.
Major and minor depression
MDD has been the focus of most drug trials seeking FDA approval. As a syndrome, MDD is defined by a constellation of features that are related not only to mood but also to sleep, energy, cognition, motivation, and motor behavior, persisting for ≥2 weeks.
DSM-5 has imposed few changes to the basic definition of MDD:
• bereavement (the aftermath of death of a loved one), formerly an exclusion criterion, no longer precludes making a diagnosis of MDD when syndromal criteria are otherwise fulfilled
• “with anxious distress” is a new course specifier that designates prominent anxiety features (feeling worried, restless, tense, or keyed up; fearful of losing control or something terrible happening)
• “with mixed features” is a new course specifier pertinent when ≥3 mania or hypomania symptoms coexist (that is, might be a subsyndromal mania or hypomania) with a depressive syndrome; the mixed features specifier can be applied to depressed patients whether or not they have ever had a manic or hypomanic episode, but MDD—rather than bipolar disorder— remains the overarching diagnosis, unless criteria have ever been met for a full mania or hypomania.
More than 2 dozen medications are FDA-approved to treat MDD. Evidence-based psychotherapies (eg, cognitive-behavioral therapy [CBT] and interpersonal therapy), as adjuncts to pharmacotherapy, further improve outcomes, but with only modest additional effect.2
Minor depression. Depressive states that involve 2 to 4 associated symptoms lasting ≥2 weeks but <2 years are sometimes described as minor depression, captured within DSM-5 as “depression not elsewhere defined.” The terminology of so-called “minor depression” generally is shunned, in part because it might wrongly connote low severity and therefore discourage treatment—even though it confers more than a 5-fold increase in risk of MDD.3
Chronicity
DSM-IV-TR identified long-standing depression by 2 constructs:
• chronic major depression (an episode of MDD lasting ≥2 years in adults, ≥1 year in children and adolescents)
• dysthymic disorder (2 to 4 depressive symptoms for ≥2 years in adults and ≥1 year in children and adolescents), affecting 3% to 6% of adults and carrying a 2-fold increased risk of MDD, eventually.4
Depression that begins as dysthymic disorder and blossoms into syndromal MDD is described as “double depression”—although it is not recognized as a unique condition in any edition of the DSM. Subsequent incomplete recovery may revert to dysthymic disorder. DSM-5 has subsumed chronic major depression and dysthymia under the unified heading of persistent depressive disorder.
There are no FDA-approved drugs for treating dysthymia. A meta-analysis of 9 controlled trials of off-label use of antidepressants to treat dysthymia revealed an overall response rate of 52.4%, compared with 29.9% for placebo.5 Notably, although the active drug response rate in these studies is comparable to what seen in MDD, the placebo response rate was approximately 10% lower than what was seen in major depression.
Positive therapeutic findings (typically, treatment for 6 to 12 weeks) have been reported in so-called “pure” dysthymic disorder with sertraline, fluoxetine, imipramine, ritanserin, moclobemide (not approved for use in the United States), and phenelzine; the results of additional, positive placebo-controlled studies support the utility of duloxetine6 and paroxetine.7 Randomized trials have reported negative findings for desipramine,8 fluoxetine,9 and escitalopram10 escitalopram10—although the sample size in these latter studies might have been too small to detect a drug-placebo difference.
In dysthymic and minor-depressive middle-age and older adult men who have a low serum level of testosterone, hormone replacement was shown to be superior to placebo in several randomized trials.11 Studies of adjunctive atypical antipsychotics for dysthymic disorder are scarce; a Cochrane review identified controlled data only with amisulpride (not approved for use in the United States), which yielded a modest therapeutic effect.12
Polarity
In recent years, depression in bipolar disorder (BD) has been contrasted with unipolar MDD based on a difference in:
• duration (briefer in BD)
• severity (worse in BD)
• risk of suicide (higher)
• comorbidity (more extensive)
• family history (often present for BD and highly recurrent depression)
• treatment outcome (generally less favorable).
DSM-5 has at least somewhat blurred the distinctions in polarity by way of the new construct of “major depression with mixed features” (see the discussion of MDD above), identifiable even when a person has never had a full manic or hypomanic episode.
No randomized trials have been conducted to identify the best treatments for such presentations, which has invited extrapolation from the literature in regard to bipolar mixed episodes, and suggesting that 1) some mood stabilizers (eg, divalproex) might have value and 2) antidepressants might exacerbate manic symptoms.
Perhaps most noteworthy in regard to treating bipolar depression is the unresolved, but hotly debated, controversy over whether and, if so, when, an antidepressant is inappropriate (based on concerns about possible induction or exacerbation of manic symptoms). In addition, nearly all of the large, randomized controlled trials of antidepressants for bipolar depression have shown that they offer no advantage over placebo.
Some authors argue that a lack of response to antidepressants might, itself, be a “soft” indicator of “bipolarity.” However, nonresponse to antidepressants should prompt a wider assessment of features other than polarity—including psychosis, anxiety, substance abuse, a personality disorder, psychiatric adverse effects from concomitant medications, medical comor bidity, adequacy of trials of medical therapy, and potential non-adherence to such trials—to account for poor antidepressant outcomes.
Severity
Severity of depression warrants consideration when formulating impressions about the nature and treatment of all presentations of depression.
High-severity forms prompt decisions about treatment setting (inpatient or outpatient); suicide assessment; and therapeutic modalities (eg, electroconvulsive therapy is more appropriate than psychotherapy for catatonic depression).
Mild forms. A recent meta-analysis of 6 randomized trials (each of >6 weeks’ duration) of antidepressants for mild depression demonstrated that these agents exert only a modest effect compared with placebo, owing largely to higher placebo-responsivity in mild depressive episodes than in moderate and severe episodes.13 In contrast, another meta-analysis of subjects who had “mild” baseline depression severity scores found that antidepressant medication had greater efficacy than placebo in 4 of 6 randomized trials.14 Higher depression severity levels typically diminish the placebo response rate but also reduce the magnitude of drug efficacy.
Psychosis
Before DSM-III, psychotic (as opposed to neurotic) depression was perhaps the key nosologic distinction when characterizing forms of depression. The presence of psychosis and related components (eg, mood-congruence) is closely linked with the severity of depression (high) and prognosis and longitudinal outcome (poorer), and has implications for treatment (Table).
Bottom Line
Depressive disorders comprise a range of conditions that can be viewed along many dimensions, including polarity, chronicity, recurrence, psychosis, treatment resistance, comorbidity, and atypicality, among other classifications. Clinical characteristics vary across subtypes—and so do corresponding preferred treatments, which should be tailored to the needs of each of your patients.
Related Resources
• Goldberg JF, Thase ME. Monoamine oxidase inhibitors revisited: what you should know. J Clin Psychiatry. 2013;74(2):189-191.
• Goldberg JF. Antidepressants in bipolar disorder: 7 myths and realities. Current Psychiatry. 2010;9(5):41-49.
• Ketamine cousin rapidly lifts depression without side effects. National Institute of Mental Health. http://www. nimh.nih.gov/news/science-news/2013/ketamine-cousin-rapidly-lifts-depression-without-side-effects.shtml. Published May 23, 2013. Accessed March 20, 2014.
• Research Domain Criteria (RDoC). National Institute of Mental Health. http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml?u tm_ source = govdelivery&utm_medium=email&utm_campaign= govdelivery. Accessed March 20, 2014.
Drug Brand Names
Amisulpride • Amazeo, Lurasidone • Latuda
Amival, Amipride, Sulpitax Mirtazapine • Remeron
Aripiprazole • Abilify Moclobemide • Amira,
Armodafinil • Nuvigil Aurorix, Clobemix,
Bupropion • Wellbutrin Depnil, Manerix
Desipramine • Norpramin Modafinil • Provigil
Divalproex • Depakote, Olanzapine/fluoxetine
Depakene • Symbyax
Duloxetine • Cymbalta Paroxetine • Paxil
Escitalopram • Lexapro Phenelzine • Nardil
Fluoxetine • Prozac Pramipexole • Mirapex
Imipramine • Tofranil Quetiapine • Seroquel
Ketamine • Ketalar Riluzole • Rilutek
Lamotrigine • Lamictal Sertraline • Zoloft
Lithium • Eskalith, Lithobid Vortioxetine • Brintellix
Disclosure
Dr. Goldberg has been a consultant to Avanir Pharmaceuticals and Merck; has served on the speakers’ bureau for AstraZeneca, Merck, Novartis, Sunovion
Pharmaceuticals, and Takeda and Lundbeck; and has received royalties from American Psychiatric Publishing and honoraria from Medscape and WebMD.
Editor’s note: The second part of Dr. Goldberg’s review of depression subtypes—focusing on “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder—will appear in the May 2014 issue of Current Psychiatry.
1. Kraepelin E. Manic-depressive insanity and paranoia. Barclay RM, trans. Robertson GM, ed. Edinburgh, Scotland: E&S Livingstone; 1921:1.
2. Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70(9):1219-1229.
3. Fogel J, Eaton WW, Ford DE. Minor depression as a predictor of the first onset of major depressive disorder over a 15-year follow-up. Acta Psychiatr Scand. 2006; 113(1):36-43.
4. Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79(1-3):71-79.
5. Levkovitz Y, Tedeschini E, Papakostas GI. Efficacy of antidepressants for dysthymia: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(4):509-514.
6. Hellerstein DJ, Stewart JW, McGrath PJ, et al. A randomized controlled trial of duloxetine versus placebo in the treatment of nonmajor chronic depression. J Clin Psychiatry. 2012;73(7):984-991.
7. Ravindran AV, Cameron C, Bhatla R, et al. Paroxetine in the treatment of dysthymic disorder without co-morbidities: a double-blind, placebo-controlled, flexible-dose study. Asian J Psychiatry. 2013;6(2):157-161.
8. Stewart JW, McGrath PJ, Liebowitz MR, et al. Treatment outcome validation of DSM-III depressive subtypes. Clinical usefulness in outpatients with mild to moderate depression. Arch Gen Psychiatry. 1985;42(12):1148-1153.
9. Serrano-Blanco A, Gabarron E, Garcia-Bayo I, et al. Effectiveness and cost-effectiveness of antidepressant treatment in primary health care: a six-month randomised study comparing fluoxetine to imipramine. J Affect Disord. 2006;91(2-3):153-163.
10. Hellerstein DJ, Batchelder ST, Hyler S, et al. Escitalopram versus placebo in the treatment of dysthymic disorders. Int Clin Psychopharmacol. 2010;25(3):143-148.
11. Seidman SN, Orr G, Raviv G, et al. Effects of testosterone replacement in middle-aged men with dysthymia: a randomized, placebo-controlled clinical trial. J Clin Psychopharmacol. 2009;29(3):216-221.
12. Komossa K, Depping AM, Gaudchau A, et al. Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. 2010; 8:(12):CD008121.
13. Fournier JC, DeRubeis RJ, Hollom SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
14. Stewart JA, Deliyannides DA, Hellerstein DJ, et al. Can people with nonsevere major depression benefit from antidepressant medication? J Clin Psychiatry. 2012;73(4):518-525.
Depression carries a wide differential diagnosis. Practitioners sometimes think polarity is the fundamental distinction when they conceptualize depression as a clinical entity; in fact, many nosologic frameworks have been described for defining and subtyping clinically meaningful forms of depression, and each waxed and waned in popularity.
Kraepelin, writing in the early 20th century, linked manic-depressive illness with “the greater part of the morbid states termed melancholia,”1 but many features other than polarity remain important components of depression, and those features often carry implications for how individual patients respond to treatment.
In this 2-part article [April and May 2014 issues], I summarize information about clinically distinct subtypes of depression, as they are recognized within diagnostic systems or as descriptors of treatment outcomes for particular subgroups of patients. My focus is on practical considerations for assessing and managing depression. Because many forms of the disorder respond inadequately to initial antidepressant treatment, optimal “next-step” pharmacotherapy, after nonresponse or partial response, often hinges on clinical subtyping.
The first part of this article examines major depressive disorder (MDD), minor depression, chronic depression, depression in bipolar disorder, depression that is severe or mild, and psychotic depression. Treatments for these subtypes for which there is evidence, or a clinical rationale, are given in the Table.
The subtypes of depression that I’ll discuss in the second part of the article are listed on page 47.
Major and minor depression
MDD has been the focus of most drug trials seeking FDA approval. As a syndrome, MDD is defined by a constellation of features that are related not only to mood but also to sleep, energy, cognition, motivation, and motor behavior, persisting for ≥2 weeks.
DSM-5 has imposed few changes to the basic definition of MDD:
• bereavement (the aftermath of death of a loved one), formerly an exclusion criterion, no longer precludes making a diagnosis of MDD when syndromal criteria are otherwise fulfilled
• “with anxious distress” is a new course specifier that designates prominent anxiety features (feeling worried, restless, tense, or keyed up; fearful of losing control or something terrible happening)
• “with mixed features” is a new course specifier pertinent when ≥3 mania or hypomania symptoms coexist (that is, might be a subsyndromal mania or hypomania) with a depressive syndrome; the mixed features specifier can be applied to depressed patients whether or not they have ever had a manic or hypomanic episode, but MDD—rather than bipolar disorder— remains the overarching diagnosis, unless criteria have ever been met for a full mania or hypomania.
More than 2 dozen medications are FDA-approved to treat MDD. Evidence-based psychotherapies (eg, cognitive-behavioral therapy [CBT] and interpersonal therapy), as adjuncts to pharmacotherapy, further improve outcomes, but with only modest additional effect.2
Minor depression. Depressive states that involve 2 to 4 associated symptoms lasting ≥2 weeks but <2 years are sometimes described as minor depression, captured within DSM-5 as “depression not elsewhere defined.” The terminology of so-called “minor depression” generally is shunned, in part because it might wrongly connote low severity and therefore discourage treatment—even though it confers more than a 5-fold increase in risk of MDD.3
Chronicity
DSM-IV-TR identified long-standing depression by 2 constructs:
• chronic major depression (an episode of MDD lasting ≥2 years in adults, ≥1 year in children and adolescents)
• dysthymic disorder (2 to 4 depressive symptoms for ≥2 years in adults and ≥1 year in children and adolescents), affecting 3% to 6% of adults and carrying a 2-fold increased risk of MDD, eventually.4
Depression that begins as dysthymic disorder and blossoms into syndromal MDD is described as “double depression”—although it is not recognized as a unique condition in any edition of the DSM. Subsequent incomplete recovery may revert to dysthymic disorder. DSM-5 has subsumed chronic major depression and dysthymia under the unified heading of persistent depressive disorder.
There are no FDA-approved drugs for treating dysthymia. A meta-analysis of 9 controlled trials of off-label use of antidepressants to treat dysthymia revealed an overall response rate of 52.4%, compared with 29.9% for placebo.5 Notably, although the active drug response rate in these studies is comparable to what seen in MDD, the placebo response rate was approximately 10% lower than what was seen in major depression.
Positive therapeutic findings (typically, treatment for 6 to 12 weeks) have been reported in so-called “pure” dysthymic disorder with sertraline, fluoxetine, imipramine, ritanserin, moclobemide (not approved for use in the United States), and phenelzine; the results of additional, positive placebo-controlled studies support the utility of duloxetine6 and paroxetine.7 Randomized trials have reported negative findings for desipramine,8 fluoxetine,9 and escitalopram10 escitalopram10—although the sample size in these latter studies might have been too small to detect a drug-placebo difference.
In dysthymic and minor-depressive middle-age and older adult men who have a low serum level of testosterone, hormone replacement was shown to be superior to placebo in several randomized trials.11 Studies of adjunctive atypical antipsychotics for dysthymic disorder are scarce; a Cochrane review identified controlled data only with amisulpride (not approved for use in the United States), which yielded a modest therapeutic effect.12
Polarity
In recent years, depression in bipolar disorder (BD) has been contrasted with unipolar MDD based on a difference in:
• duration (briefer in BD)
• severity (worse in BD)
• risk of suicide (higher)
• comorbidity (more extensive)
• family history (often present for BD and highly recurrent depression)
• treatment outcome (generally less favorable).
DSM-5 has at least somewhat blurred the distinctions in polarity by way of the new construct of “major depression with mixed features” (see the discussion of MDD above), identifiable even when a person has never had a full manic or hypomanic episode.
No randomized trials have been conducted to identify the best treatments for such presentations, which has invited extrapolation from the literature in regard to bipolar mixed episodes, and suggesting that 1) some mood stabilizers (eg, divalproex) might have value and 2) antidepressants might exacerbate manic symptoms.
Perhaps most noteworthy in regard to treating bipolar depression is the unresolved, but hotly debated, controversy over whether and, if so, when, an antidepressant is inappropriate (based on concerns about possible induction or exacerbation of manic symptoms). In addition, nearly all of the large, randomized controlled trials of antidepressants for bipolar depression have shown that they offer no advantage over placebo.
Some authors argue that a lack of response to antidepressants might, itself, be a “soft” indicator of “bipolarity.” However, nonresponse to antidepressants should prompt a wider assessment of features other than polarity—including psychosis, anxiety, substance abuse, a personality disorder, psychiatric adverse effects from concomitant medications, medical comor bidity, adequacy of trials of medical therapy, and potential non-adherence to such trials—to account for poor antidepressant outcomes.
Severity
Severity of depression warrants consideration when formulating impressions about the nature and treatment of all presentations of depression.
High-severity forms prompt decisions about treatment setting (inpatient or outpatient); suicide assessment; and therapeutic modalities (eg, electroconvulsive therapy is more appropriate than psychotherapy for catatonic depression).
Mild forms. A recent meta-analysis of 6 randomized trials (each of >6 weeks’ duration) of antidepressants for mild depression demonstrated that these agents exert only a modest effect compared with placebo, owing largely to higher placebo-responsivity in mild depressive episodes than in moderate and severe episodes.13 In contrast, another meta-analysis of subjects who had “mild” baseline depression severity scores found that antidepressant medication had greater efficacy than placebo in 4 of 6 randomized trials.14 Higher depression severity levels typically diminish the placebo response rate but also reduce the magnitude of drug efficacy.
Psychosis
Before DSM-III, psychotic (as opposed to neurotic) depression was perhaps the key nosologic distinction when characterizing forms of depression. The presence of psychosis and related components (eg, mood-congruence) is closely linked with the severity of depression (high) and prognosis and longitudinal outcome (poorer), and has implications for treatment (Table).
Bottom Line
Depressive disorders comprise a range of conditions that can be viewed along many dimensions, including polarity, chronicity, recurrence, psychosis, treatment resistance, comorbidity, and atypicality, among other classifications. Clinical characteristics vary across subtypes—and so do corresponding preferred treatments, which should be tailored to the needs of each of your patients.
Related Resources
• Goldberg JF, Thase ME. Monoamine oxidase inhibitors revisited: what you should know. J Clin Psychiatry. 2013;74(2):189-191.
• Goldberg JF. Antidepressants in bipolar disorder: 7 myths and realities. Current Psychiatry. 2010;9(5):41-49.
• Ketamine cousin rapidly lifts depression without side effects. National Institute of Mental Health. http://www. nimh.nih.gov/news/science-news/2013/ketamine-cousin-rapidly-lifts-depression-without-side-effects.shtml. Published May 23, 2013. Accessed March 20, 2014.
• Research Domain Criteria (RDoC). National Institute of Mental Health. http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml?u tm_ source = govdelivery&utm_medium=email&utm_campaign= govdelivery. Accessed March 20, 2014.
Drug Brand Names
Amisulpride • Amazeo, Lurasidone • Latuda
Amival, Amipride, Sulpitax Mirtazapine • Remeron
Aripiprazole • Abilify Moclobemide • Amira,
Armodafinil • Nuvigil Aurorix, Clobemix,
Bupropion • Wellbutrin Depnil, Manerix
Desipramine • Norpramin Modafinil • Provigil
Divalproex • Depakote, Olanzapine/fluoxetine
Depakene • Symbyax
Duloxetine • Cymbalta Paroxetine • Paxil
Escitalopram • Lexapro Phenelzine • Nardil
Fluoxetine • Prozac Pramipexole • Mirapex
Imipramine • Tofranil Quetiapine • Seroquel
Ketamine • Ketalar Riluzole • Rilutek
Lamotrigine • Lamictal Sertraline • Zoloft
Lithium • Eskalith, Lithobid Vortioxetine • Brintellix
Disclosure
Dr. Goldberg has been a consultant to Avanir Pharmaceuticals and Merck; has served on the speakers’ bureau for AstraZeneca, Merck, Novartis, Sunovion
Pharmaceuticals, and Takeda and Lundbeck; and has received royalties from American Psychiatric Publishing and honoraria from Medscape and WebMD.
Editor’s note: The second part of Dr. Goldberg’s review of depression subtypes—focusing on “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder—will appear in the May 2014 issue of Current Psychiatry.
Depression carries a wide differential diagnosis. Practitioners sometimes think polarity is the fundamental distinction when they conceptualize depression as a clinical entity; in fact, many nosologic frameworks have been described for defining and subtyping clinically meaningful forms of depression, and each waxed and waned in popularity.
Kraepelin, writing in the early 20th century, linked manic-depressive illness with “the greater part of the morbid states termed melancholia,”1 but many features other than polarity remain important components of depression, and those features often carry implications for how individual patients respond to treatment.
In this 2-part article [April and May 2014 issues], I summarize information about clinically distinct subtypes of depression, as they are recognized within diagnostic systems or as descriptors of treatment outcomes for particular subgroups of patients. My focus is on practical considerations for assessing and managing depression. Because many forms of the disorder respond inadequately to initial antidepressant treatment, optimal “next-step” pharmacotherapy, after nonresponse or partial response, often hinges on clinical subtyping.
The first part of this article examines major depressive disorder (MDD), minor depression, chronic depression, depression in bipolar disorder, depression that is severe or mild, and psychotic depression. Treatments for these subtypes for which there is evidence, or a clinical rationale, are given in the Table.
The subtypes of depression that I’ll discuss in the second part of the article are listed on page 47.
Major and minor depression
MDD has been the focus of most drug trials seeking FDA approval. As a syndrome, MDD is defined by a constellation of features that are related not only to mood but also to sleep, energy, cognition, motivation, and motor behavior, persisting for ≥2 weeks.
DSM-5 has imposed few changes to the basic definition of MDD:
• bereavement (the aftermath of death of a loved one), formerly an exclusion criterion, no longer precludes making a diagnosis of MDD when syndromal criteria are otherwise fulfilled
• “with anxious distress” is a new course specifier that designates prominent anxiety features (feeling worried, restless, tense, or keyed up; fearful of losing control or something terrible happening)
• “with mixed features” is a new course specifier pertinent when ≥3 mania or hypomania symptoms coexist (that is, might be a subsyndromal mania or hypomania) with a depressive syndrome; the mixed features specifier can be applied to depressed patients whether or not they have ever had a manic or hypomanic episode, but MDD—rather than bipolar disorder— remains the overarching diagnosis, unless criteria have ever been met for a full mania or hypomania.
More than 2 dozen medications are FDA-approved to treat MDD. Evidence-based psychotherapies (eg, cognitive-behavioral therapy [CBT] and interpersonal therapy), as adjuncts to pharmacotherapy, further improve outcomes, but with only modest additional effect.2
Minor depression. Depressive states that involve 2 to 4 associated symptoms lasting ≥2 weeks but <2 years are sometimes described as minor depression, captured within DSM-5 as “depression not elsewhere defined.” The terminology of so-called “minor depression” generally is shunned, in part because it might wrongly connote low severity and therefore discourage treatment—even though it confers more than a 5-fold increase in risk of MDD.3
Chronicity
DSM-IV-TR identified long-standing depression by 2 constructs:
• chronic major depression (an episode of MDD lasting ≥2 years in adults, ≥1 year in children and adolescents)
• dysthymic disorder (2 to 4 depressive symptoms for ≥2 years in adults and ≥1 year in children and adolescents), affecting 3% to 6% of adults and carrying a 2-fold increased risk of MDD, eventually.4
Depression that begins as dysthymic disorder and blossoms into syndromal MDD is described as “double depression”—although it is not recognized as a unique condition in any edition of the DSM. Subsequent incomplete recovery may revert to dysthymic disorder. DSM-5 has subsumed chronic major depression and dysthymia under the unified heading of persistent depressive disorder.
There are no FDA-approved drugs for treating dysthymia. A meta-analysis of 9 controlled trials of off-label use of antidepressants to treat dysthymia revealed an overall response rate of 52.4%, compared with 29.9% for placebo.5 Notably, although the active drug response rate in these studies is comparable to what seen in MDD, the placebo response rate was approximately 10% lower than what was seen in major depression.
Positive therapeutic findings (typically, treatment for 6 to 12 weeks) have been reported in so-called “pure” dysthymic disorder with sertraline, fluoxetine, imipramine, ritanserin, moclobemide (not approved for use in the United States), and phenelzine; the results of additional, positive placebo-controlled studies support the utility of duloxetine6 and paroxetine.7 Randomized trials have reported negative findings for desipramine,8 fluoxetine,9 and escitalopram10 escitalopram10—although the sample size in these latter studies might have been too small to detect a drug-placebo difference.
In dysthymic and minor-depressive middle-age and older adult men who have a low serum level of testosterone, hormone replacement was shown to be superior to placebo in several randomized trials.11 Studies of adjunctive atypical antipsychotics for dysthymic disorder are scarce; a Cochrane review identified controlled data only with amisulpride (not approved for use in the United States), which yielded a modest therapeutic effect.12
Polarity
In recent years, depression in bipolar disorder (BD) has been contrasted with unipolar MDD based on a difference in:
• duration (briefer in BD)
• severity (worse in BD)
• risk of suicide (higher)
• comorbidity (more extensive)
• family history (often present for BD and highly recurrent depression)
• treatment outcome (generally less favorable).
DSM-5 has at least somewhat blurred the distinctions in polarity by way of the new construct of “major depression with mixed features” (see the discussion of MDD above), identifiable even when a person has never had a full manic or hypomanic episode.
No randomized trials have been conducted to identify the best treatments for such presentations, which has invited extrapolation from the literature in regard to bipolar mixed episodes, and suggesting that 1) some mood stabilizers (eg, divalproex) might have value and 2) antidepressants might exacerbate manic symptoms.
Perhaps most noteworthy in regard to treating bipolar depression is the unresolved, but hotly debated, controversy over whether and, if so, when, an antidepressant is inappropriate (based on concerns about possible induction or exacerbation of manic symptoms). In addition, nearly all of the large, randomized controlled trials of antidepressants for bipolar depression have shown that they offer no advantage over placebo.
Some authors argue that a lack of response to antidepressants might, itself, be a “soft” indicator of “bipolarity.” However, nonresponse to antidepressants should prompt a wider assessment of features other than polarity—including psychosis, anxiety, substance abuse, a personality disorder, psychiatric adverse effects from concomitant medications, medical comor bidity, adequacy of trials of medical therapy, and potential non-adherence to such trials—to account for poor antidepressant outcomes.
Severity
Severity of depression warrants consideration when formulating impressions about the nature and treatment of all presentations of depression.
High-severity forms prompt decisions about treatment setting (inpatient or outpatient); suicide assessment; and therapeutic modalities (eg, electroconvulsive therapy is more appropriate than psychotherapy for catatonic depression).
Mild forms. A recent meta-analysis of 6 randomized trials (each of >6 weeks’ duration) of antidepressants for mild depression demonstrated that these agents exert only a modest effect compared with placebo, owing largely to higher placebo-responsivity in mild depressive episodes than in moderate and severe episodes.13 In contrast, another meta-analysis of subjects who had “mild” baseline depression severity scores found that antidepressant medication had greater efficacy than placebo in 4 of 6 randomized trials.14 Higher depression severity levels typically diminish the placebo response rate but also reduce the magnitude of drug efficacy.
Psychosis
Before DSM-III, psychotic (as opposed to neurotic) depression was perhaps the key nosologic distinction when characterizing forms of depression. The presence of psychosis and related components (eg, mood-congruence) is closely linked with the severity of depression (high) and prognosis and longitudinal outcome (poorer), and has implications for treatment (Table).
Bottom Line
Depressive disorders comprise a range of conditions that can be viewed along many dimensions, including polarity, chronicity, recurrence, psychosis, treatment resistance, comorbidity, and atypicality, among other classifications. Clinical characteristics vary across subtypes—and so do corresponding preferred treatments, which should be tailored to the needs of each of your patients.
Related Resources
• Goldberg JF, Thase ME. Monoamine oxidase inhibitors revisited: what you should know. J Clin Psychiatry. 2013;74(2):189-191.
• Goldberg JF. Antidepressants in bipolar disorder: 7 myths and realities. Current Psychiatry. 2010;9(5):41-49.
• Ketamine cousin rapidly lifts depression without side effects. National Institute of Mental Health. http://www. nimh.nih.gov/news/science-news/2013/ketamine-cousin-rapidly-lifts-depression-without-side-effects.shtml. Published May 23, 2013. Accessed March 20, 2014.
• Research Domain Criteria (RDoC). National Institute of Mental Health. http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml?u tm_ source = govdelivery&utm_medium=email&utm_campaign= govdelivery. Accessed March 20, 2014.
Drug Brand Names
Amisulpride • Amazeo, Lurasidone • Latuda
Amival, Amipride, Sulpitax Mirtazapine • Remeron
Aripiprazole • Abilify Moclobemide • Amira,
Armodafinil • Nuvigil Aurorix, Clobemix,
Bupropion • Wellbutrin Depnil, Manerix
Desipramine • Norpramin Modafinil • Provigil
Divalproex • Depakote, Olanzapine/fluoxetine
Depakene • Symbyax
Duloxetine • Cymbalta Paroxetine • Paxil
Escitalopram • Lexapro Phenelzine • Nardil
Fluoxetine • Prozac Pramipexole • Mirapex
Imipramine • Tofranil Quetiapine • Seroquel
Ketamine • Ketalar Riluzole • Rilutek
Lamotrigine • Lamictal Sertraline • Zoloft
Lithium • Eskalith, Lithobid Vortioxetine • Brintellix
Disclosure
Dr. Goldberg has been a consultant to Avanir Pharmaceuticals and Merck; has served on the speakers’ bureau for AstraZeneca, Merck, Novartis, Sunovion
Pharmaceuticals, and Takeda and Lundbeck; and has received royalties from American Psychiatric Publishing and honoraria from Medscape and WebMD.
Editor’s note: The second part of Dr. Goldberg’s review of depression subtypes—focusing on “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder—will appear in the May 2014 issue of Current Psychiatry.
1. Kraepelin E. Manic-depressive insanity and paranoia. Barclay RM, trans. Robertson GM, ed. Edinburgh, Scotland: E&S Livingstone; 1921:1.
2. Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70(9):1219-1229.
3. Fogel J, Eaton WW, Ford DE. Minor depression as a predictor of the first onset of major depressive disorder over a 15-year follow-up. Acta Psychiatr Scand. 2006; 113(1):36-43.
4. Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79(1-3):71-79.
5. Levkovitz Y, Tedeschini E, Papakostas GI. Efficacy of antidepressants for dysthymia: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(4):509-514.
6. Hellerstein DJ, Stewart JW, McGrath PJ, et al. A randomized controlled trial of duloxetine versus placebo in the treatment of nonmajor chronic depression. J Clin Psychiatry. 2012;73(7):984-991.
7. Ravindran AV, Cameron C, Bhatla R, et al. Paroxetine in the treatment of dysthymic disorder without co-morbidities: a double-blind, placebo-controlled, flexible-dose study. Asian J Psychiatry. 2013;6(2):157-161.
8. Stewart JW, McGrath PJ, Liebowitz MR, et al. Treatment outcome validation of DSM-III depressive subtypes. Clinical usefulness in outpatients with mild to moderate depression. Arch Gen Psychiatry. 1985;42(12):1148-1153.
9. Serrano-Blanco A, Gabarron E, Garcia-Bayo I, et al. Effectiveness and cost-effectiveness of antidepressant treatment in primary health care: a six-month randomised study comparing fluoxetine to imipramine. J Affect Disord. 2006;91(2-3):153-163.
10. Hellerstein DJ, Batchelder ST, Hyler S, et al. Escitalopram versus placebo in the treatment of dysthymic disorders. Int Clin Psychopharmacol. 2010;25(3):143-148.
11. Seidman SN, Orr G, Raviv G, et al. Effects of testosterone replacement in middle-aged men with dysthymia: a randomized, placebo-controlled clinical trial. J Clin Psychopharmacol. 2009;29(3):216-221.
12. Komossa K, Depping AM, Gaudchau A, et al. Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. 2010; 8:(12):CD008121.
13. Fournier JC, DeRubeis RJ, Hollom SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
14. Stewart JA, Deliyannides DA, Hellerstein DJ, et al. Can people with nonsevere major depression benefit from antidepressant medication? J Clin Psychiatry. 2012;73(4):518-525.
1. Kraepelin E. Manic-depressive insanity and paranoia. Barclay RM, trans. Robertson GM, ed. Edinburgh, Scotland: E&S Livingstone; 1921:1.
2. Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70(9):1219-1229.
3. Fogel J, Eaton WW, Ford DE. Minor depression as a predictor of the first onset of major depressive disorder over a 15-year follow-up. Acta Psychiatr Scand. 2006; 113(1):36-43.
4. Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79(1-3):71-79.
5. Levkovitz Y, Tedeschini E, Papakostas GI. Efficacy of antidepressants for dysthymia: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(4):509-514.
6. Hellerstein DJ, Stewart JW, McGrath PJ, et al. A randomized controlled trial of duloxetine versus placebo in the treatment of nonmajor chronic depression. J Clin Psychiatry. 2012;73(7):984-991.
7. Ravindran AV, Cameron C, Bhatla R, et al. Paroxetine in the treatment of dysthymic disorder without co-morbidities: a double-blind, placebo-controlled, flexible-dose study. Asian J Psychiatry. 2013;6(2):157-161.
8. Stewart JW, McGrath PJ, Liebowitz MR, et al. Treatment outcome validation of DSM-III depressive subtypes. Clinical usefulness in outpatients with mild to moderate depression. Arch Gen Psychiatry. 1985;42(12):1148-1153.
9. Serrano-Blanco A, Gabarron E, Garcia-Bayo I, et al. Effectiveness and cost-effectiveness of antidepressant treatment in primary health care: a six-month randomised study comparing fluoxetine to imipramine. J Affect Disord. 2006;91(2-3):153-163.
10. Hellerstein DJ, Batchelder ST, Hyler S, et al. Escitalopram versus placebo in the treatment of dysthymic disorders. Int Clin Psychopharmacol. 2010;25(3):143-148.
11. Seidman SN, Orr G, Raviv G, et al. Effects of testosterone replacement in middle-aged men with dysthymia: a randomized, placebo-controlled clinical trial. J Clin Psychopharmacol. 2009;29(3):216-221.
12. Komossa K, Depping AM, Gaudchau A, et al. Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. 2010; 8:(12):CD008121.
13. Fournier JC, DeRubeis RJ, Hollom SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
14. Stewart JA, Deliyannides DA, Hellerstein DJ, et al. Can people with nonsevere major depression benefit from antidepressant medication? J Clin Psychiatry. 2012;73(4):518-525.