User login
A counterfeit version of the “morning after pill” may be in distribution in the United States, specifically in some Hispanic communities. The US Food and Drug Administration (FDA) has not approved Evital for use, and it may not be safe or effective in preventing pregnancy.
The packaging label of this potentially ineffective and suspect counterfeit emergency birth control says, “Evital Anticonceptivo de emergencia, 1.5 mg, 1 tablet”, by “Fluter Domull” (FIGURE).
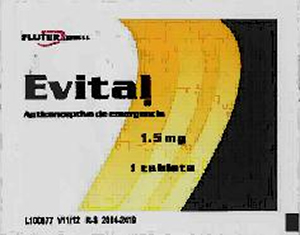
Consumers should not take the Evital product if its packaging looks like the FIGURE. If consumers have this product, they should:
- Contact their doctor or health care professional if they have taken Evital labeled as the 1.5 mg tablet and experienced any problems.
- Talk with a doctor, pharmacist, or health care professional to find the FDA-approved emergency birth control medicine best for you.
- There are FDA-approved emergency birth control medicines available both with a prescription, and over-the-counter without a prescription (if you are 17 years old or older).
The FDA is asking for help from anyone who has information about Evital. Please send an e-mail to CDER_Ingredient_Adulteration@fda.hhs.gov to provide information or ask additional questions. Any information received is confidential and will be used only to help in FDA’s effort to remove the potentially unsafe and ineffective versions from the US marketplace.
Reference
1. Unapproved emergency birth control medicine possibly in U.S. distribution may be ineffective and unsafe [press release]. US Food and Drug Administration Web site. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm265847.htm. Published July 28, 2011. Accessed August 3, 2011.
A counterfeit version of the “morning after pill” may be in distribution in the United States, specifically in some Hispanic communities. The US Food and Drug Administration (FDA) has not approved Evital for use, and it may not be safe or effective in preventing pregnancy.
The packaging label of this potentially ineffective and suspect counterfeit emergency birth control says, “Evital Anticonceptivo de emergencia, 1.5 mg, 1 tablet”, by “Fluter Domull” (FIGURE).

Consumers should not take the Evital product if its packaging looks like the FIGURE. If consumers have this product, they should:
- Contact their doctor or health care professional if they have taken Evital labeled as the 1.5 mg tablet and experienced any problems.
- Talk with a doctor, pharmacist, or health care professional to find the FDA-approved emergency birth control medicine best for you.
- There are FDA-approved emergency birth control medicines available both with a prescription, and over-the-counter without a prescription (if you are 17 years old or older).
The FDA is asking for help from anyone who has information about Evital. Please send an e-mail to CDER_Ingredient_Adulteration@fda.hhs.gov to provide information or ask additional questions. Any information received is confidential and will be used only to help in FDA’s effort to remove the potentially unsafe and ineffective versions from the US marketplace.
A counterfeit version of the “morning after pill” may be in distribution in the United States, specifically in some Hispanic communities. The US Food and Drug Administration (FDA) has not approved Evital for use, and it may not be safe or effective in preventing pregnancy.
The packaging label of this potentially ineffective and suspect counterfeit emergency birth control says, “Evital Anticonceptivo de emergencia, 1.5 mg, 1 tablet”, by “Fluter Domull” (FIGURE).

Consumers should not take the Evital product if its packaging looks like the FIGURE. If consumers have this product, they should:
- Contact their doctor or health care professional if they have taken Evital labeled as the 1.5 mg tablet and experienced any problems.
- Talk with a doctor, pharmacist, or health care professional to find the FDA-approved emergency birth control medicine best for you.
- There are FDA-approved emergency birth control medicines available both with a prescription, and over-the-counter without a prescription (if you are 17 years old or older).
The FDA is asking for help from anyone who has information about Evital. Please send an e-mail to CDER_Ingredient_Adulteration@fda.hhs.gov to provide information or ask additional questions. Any information received is confidential and will be used only to help in FDA’s effort to remove the potentially unsafe and ineffective versions from the US marketplace.
Reference
1. Unapproved emergency birth control medicine possibly in U.S. distribution may be ineffective and unsafe [press release]. US Food and Drug Administration Web site. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm265847.htm. Published July 28, 2011. Accessed August 3, 2011.
Reference
1. Unapproved emergency birth control medicine possibly in U.S. distribution may be ineffective and unsafe [press release]. US Food and Drug Administration Web site. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm265847.htm. Published July 28, 2011. Accessed August 3, 2011.