User login
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7-9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
Methods
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
Results
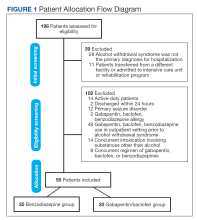
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28).
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome.
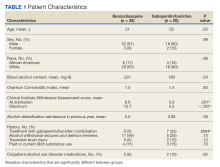
Additionally, this study examined multiple secondary outcomes (Table 2).
Discussion
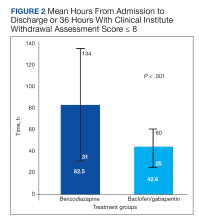
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate recordkeeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
Conclusions
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
1. National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. Updated April 2021. Accessed February 2, 2023. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder
2. Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3-4):175-177. doi:10.1080/19371918.2013.758987
3. Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. Accessed January 26, 2023. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis
4. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
5. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144-151. doi:10.1001/jama.278.2.144
6. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649-655.
7. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi:10.1111/j.1530-0277.2009.00986.x
8. Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics. 2018;59(5):496-505. doi:10.1016/j.psym.2018.03.002
9. Bates RE, Leung JG, Morgan RJ 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542-549. Published 2020 Aug 19. doi:10.1016/j.mayocpiqo.2020.06.002
10. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
11. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. doi:10.1177/1060028015585849
12. Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi:10.3389/fpsyt.2018.00773
13. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. Published 2019 Nov 6. doi:10.1002/14651858.CD008502.pub6
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7-9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
Methods
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
Results
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28).
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome.
Additionally, this study examined multiple secondary outcomes (Table 2).
Discussion
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate recordkeeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
Conclusions
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7-9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
Methods
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
Results
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28).
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome.
Additionally, this study examined multiple secondary outcomes (Table 2).
Discussion
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate recordkeeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
Conclusions
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
1. National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. Updated April 2021. Accessed February 2, 2023. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder
2. Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3-4):175-177. doi:10.1080/19371918.2013.758987
3. Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. Accessed January 26, 2023. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis
4. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
5. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144-151. doi:10.1001/jama.278.2.144
6. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649-655.
7. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi:10.1111/j.1530-0277.2009.00986.x
8. Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics. 2018;59(5):496-505. doi:10.1016/j.psym.2018.03.002
9. Bates RE, Leung JG, Morgan RJ 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542-549. Published 2020 Aug 19. doi:10.1016/j.mayocpiqo.2020.06.002
10. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
11. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. doi:10.1177/1060028015585849
12. Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi:10.3389/fpsyt.2018.00773
13. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. Published 2019 Nov 6. doi:10.1002/14651858.CD008502.pub6
1. National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. Updated April 2021. Accessed February 2, 2023. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder
2. Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3-4):175-177. doi:10.1080/19371918.2013.758987
3. Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. Accessed January 26, 2023. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis
4. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
5. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144-151. doi:10.1001/jama.278.2.144
6. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649-655.
7. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi:10.1111/j.1530-0277.2009.00986.x
8. Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics. 2018;59(5):496-505. doi:10.1016/j.psym.2018.03.002
9. Bates RE, Leung JG, Morgan RJ 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542-549. Published 2020 Aug 19. doi:10.1016/j.mayocpiqo.2020.06.002
10. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
11. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. doi:10.1177/1060028015585849
12. Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi:10.3389/fpsyt.2018.00773
13. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. Published 2019 Nov 6. doi:10.1002/14651858.CD008502.pub6