User login
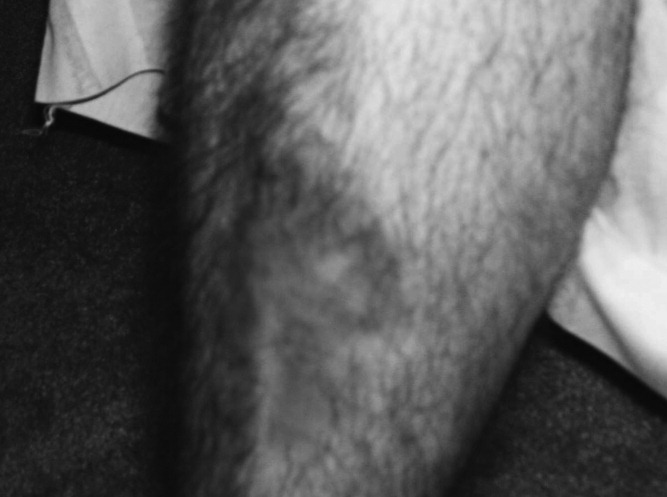
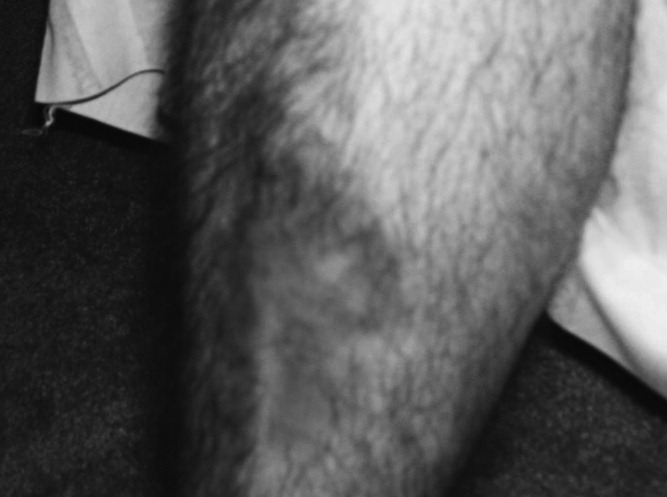
A 34‐year‐old man was admitted for evaluation of elevated blood glucose despite extremely high subcutaneous (SQ) insulin requirements. He had a 12‐year history of Type 2 diabetes mellitus (T2DM) without episodes of ketoacidosis, managed initially with oral medications (metformin with various sulfonylureas and thiazolidinediones). Three months prior to admission, he was transitioned to SQ insulin and thereafter his requirements escalated rapidly. By the time of his admission, his blood glucose measurements were consistently above 300 mg/dL despite injecting more than 4100 units of insulin daily. His regimen included 300 units of insulin glargine (Lantus) 2 times per day (BID) and 1.75 mL of Humilin U‐500 Insulin (875 units) 4 times per day (QID). Past medical history included metabolic syndrome, nonalcoholic steatohepatitis, and diabetic neuropathy. Physical exam was remarkable for centripetal obesity (body mass index [BMI] = 38.9 kg/m2), acanthosis nigricans, and necrobiosis lipoidica diabeticorum (NLD) (Figure 1).
We undertook an investigation to characterize this extreme insulin resistance. After 24 hours without insulin supplementation, and 12 hours of nothing by mouth (NPO), his blood glucose level was 280 mg/dL and his serum insulin was 133.5 IU/mL. We injected 12 units of insulin Aspart and subsequently measured his serum glucose and insulin once more. His blood glucose level had risen to 289 mg/dL and his serum insulin fell to 110.7 IU/mL. We then transitioned the patient to intravenous (IV) insulin. After a series of boluses totaling 400 units, his blood glucose normalized (90 mg/dL) and was maintained in normal range on a rate of 48 units per hour. Over 24 hours, we had infused over 1400 units.
During this time, we also drew several labs. Serum antiinsulin antibodies were undetectable (ARUP Laboratories, Salt Lake City, UT). A full rheumatologic workup was negative for systemic lupus erythematosus (SLE), rheumatoid factor, Sjgren's syndrome (SS)‐A and SS‐B. Androgen levels were normal, as were 24‐hour urine collections for cortisol and metanephrines. The patient was discharged on a regimen of U‐500 without glargine.
By 5 months after discharge, his blood glucose remained uncontrolled despite increasing doses of U‐500 (with or without metformin and thiazolidinediones). The patient was offered a gastric bypass operation. Now, 4 months postoperative, his blood glucose is controlled, no greater than 90 mg/dL in the morning and 125 mg/dL in the evening. He is off insulin, taking 30 mg pioglitazone (Actos) daily and 500 mg metformin 3 times per day (TID).
Discussion
Extreme insulin resistance (EIR), defined by daily insulin requirements in excess of 200 U, is a rare and frustrating condition.1 Rarer still is extreme subcutaneous insulin resistance (ESIR). A systematic Medline review revealed only 29 reported cases of ESIR, all of which involved patients that maintained IV sensitivity to insulin. Classic diagnostic criteria for ESIR include preserved sensitivity to IV insulin, failure to increase serum insulin with subcutaneous injection, and insulin degrading activity of subcutaneous tissue.2, 3 However, there are, at present, no laboratory tests that can test the final criterion. Indeed, very few of the published reports of ESIR satisfy it, with most studies considering as diagnostic of ESIR the constellation of EIR with failure to raise serum insulin after injection and preserved intravenous insulin sensitivity.
As was evident in the high doses of IV insulin required for blood glucose normalization, our patient also had a proven receptor‐level peripheral resistance. Beyond the common, multifactorial insulin resistance of T2DM, the published reports of patients with extreme peripheral resistance are of 2 types: (A) genetic (eg, Leprechaunism) and (B) acquired autoimmune (Table 1).4 This patient fits neither category. Patients with Type A are very sick, with a syndromic disease that sharply curtails their life expectancy. Patients with Type B acquire antibodies directed against their insulin receptors and are almost invariably elderly African‐American women with severe rheumatological disease, namely SLE. We could not test our patient for an insulin‐receptor antibody secondary to prohibitive cost. This is probably moot, given that his autoimmune workup was negative and, as above, patients with such antibodies are vastly different compared to our patients.
| Class of Insulin Resistance | Mechanism | Incidence | Treatment |
|---|---|---|---|
| |||
| Type 2 diabetes mellitus | Multifactorial | 3% of total population | Many |
| Type A receptor‐level insulin resistance | Congenital receptor defect | 86 cases | U‐500, insulin‐like growth factor‐1 |
| Type B receptor‐level insulin resistance | Antiinsulin receptor antibody | 50 cases | U‐500, immune modulation |
| Subcutaneous insulin resistance | Unknown; SQ protease? | 30 cases | U‐500, intraperitoneal insulin delivery, other |
Based on SQ insulin requirements, our patient had EIR. As his insulin levels failed to rise following an insulin injection, his EIR is thus subcutaneous in nature. However, among patients with this condition his failure to respond to IV insulin is unique. He does not fit criteria for types A or B insulin resistance; his condition is likely also due to an extreme version of the more common, multifactorial peripheral insulin resistance. This is supported by his successful response to the gastric bypass operation.5
The standard treatments for ESIR include: (1) concentrated regular insulin (U‐500) and (2) implantable intraperitoneal delivery; our patient received the former.6 U‐500 use in EIR has been shown to be more cost‐effective.1 Several reports have suggested success with protease inhibitors (aprotinin, nafamostat ointment), plasmapheresis, and intravenous immunoglobulin for extreme SQ resistance. Our case also represents the first treated successfully with a gastric bypass operation.
CONCLUSIONS
EIR can present a significant challenge for both the patient and hospitalist. The approach to this condition should begin with the determination of 24‐hour IV insulin requirement utilizing an insulin drip; serum insulin antibody evaluation; and endocrinology consultation. Our case also highlights a few important points about the broader management of diabetes mellitus. First, there are dermatological manifestations of diabetes that serve as potential markers for disease (namely acanthosis nigricans and NLD). Second, for patients with extreme insulin requirements, an extensive workup should be initiated and the patient should be transitioned to a concentrated regular insulin or intraperitoneal delivery. Third, our experience suggests a role for other measures such as gastric bypass that ought to be studied further.
- ,,.The use of U‐500 in patients with extreme insulin resistance.Diabetes Care.2005;28:1240–1244.
- ,.Impaired absorption of insulin as a cause insulin resistance.Diabetes.1975;24:443.
- ,,.Insulin resistance caused by massive degradation of subcutaneous insulin.Diabetes.1979;28:640–645.
- ,,, et al.Clinical course of genetic diseases of the insulin receptor: a 30‐year prospective.Medicine.2004;83:209–222.
- ,,, et al.Who would have thought it? An operation proves to be the most effective therapy for adult‐onset diabetes mellitus.Ann Surg.1995;222:339–352.
- ,,,,,.Extreme subcutaneous insulin resistance: a misunderstood syndrome.Diabetes Metab.2003;29:539–546.
A 34‐year‐old man was admitted for evaluation of elevated blood glucose despite extremely high subcutaneous (SQ) insulin requirements. He had a 12‐year history of Type 2 diabetes mellitus (T2DM) without episodes of ketoacidosis, managed initially with oral medications (metformin with various sulfonylureas and thiazolidinediones). Three months prior to admission, he was transitioned to SQ insulin and thereafter his requirements escalated rapidly. By the time of his admission, his blood glucose measurements were consistently above 300 mg/dL despite injecting more than 4100 units of insulin daily. His regimen included 300 units of insulin glargine (Lantus) 2 times per day (BID) and 1.75 mL of Humilin U‐500 Insulin (875 units) 4 times per day (QID). Past medical history included metabolic syndrome, nonalcoholic steatohepatitis, and diabetic neuropathy. Physical exam was remarkable for centripetal obesity (body mass index [BMI] = 38.9 kg/m2), acanthosis nigricans, and necrobiosis lipoidica diabeticorum (NLD) (Figure 1).

We undertook an investigation to characterize this extreme insulin resistance. After 24 hours without insulin supplementation, and 12 hours of nothing by mouth (NPO), his blood glucose level was 280 mg/dL and his serum insulin was 133.5 IU/mL. We injected 12 units of insulin Aspart and subsequently measured his serum glucose and insulin once more. His blood glucose level had risen to 289 mg/dL and his serum insulin fell to 110.7 IU/mL. We then transitioned the patient to intravenous (IV) insulin. After a series of boluses totaling 400 units, his blood glucose normalized (90 mg/dL) and was maintained in normal range on a rate of 48 units per hour. Over 24 hours, we had infused over 1400 units.
During this time, we also drew several labs. Serum antiinsulin antibodies were undetectable (ARUP Laboratories, Salt Lake City, UT). A full rheumatologic workup was negative for systemic lupus erythematosus (SLE), rheumatoid factor, Sjgren's syndrome (SS)‐A and SS‐B. Androgen levels were normal, as were 24‐hour urine collections for cortisol and metanephrines. The patient was discharged on a regimen of U‐500 without glargine.
By 5 months after discharge, his blood glucose remained uncontrolled despite increasing doses of U‐500 (with or without metformin and thiazolidinediones). The patient was offered a gastric bypass operation. Now, 4 months postoperative, his blood glucose is controlled, no greater than 90 mg/dL in the morning and 125 mg/dL in the evening. He is off insulin, taking 30 mg pioglitazone (Actos) daily and 500 mg metformin 3 times per day (TID).
Discussion
Extreme insulin resistance (EIR), defined by daily insulin requirements in excess of 200 U, is a rare and frustrating condition.1 Rarer still is extreme subcutaneous insulin resistance (ESIR). A systematic Medline review revealed only 29 reported cases of ESIR, all of which involved patients that maintained IV sensitivity to insulin. Classic diagnostic criteria for ESIR include preserved sensitivity to IV insulin, failure to increase serum insulin with subcutaneous injection, and insulin degrading activity of subcutaneous tissue.2, 3 However, there are, at present, no laboratory tests that can test the final criterion. Indeed, very few of the published reports of ESIR satisfy it, with most studies considering as diagnostic of ESIR the constellation of EIR with failure to raise serum insulin after injection and preserved intravenous insulin sensitivity.
As was evident in the high doses of IV insulin required for blood glucose normalization, our patient also had a proven receptor‐level peripheral resistance. Beyond the common, multifactorial insulin resistance of T2DM, the published reports of patients with extreme peripheral resistance are of 2 types: (A) genetic (eg, Leprechaunism) and (B) acquired autoimmune (Table 1).4 This patient fits neither category. Patients with Type A are very sick, with a syndromic disease that sharply curtails their life expectancy. Patients with Type B acquire antibodies directed against their insulin receptors and are almost invariably elderly African‐American women with severe rheumatological disease, namely SLE. We could not test our patient for an insulin‐receptor antibody secondary to prohibitive cost. This is probably moot, given that his autoimmune workup was negative and, as above, patients with such antibodies are vastly different compared to our patients.
| Class of Insulin Resistance | Mechanism | Incidence | Treatment |
|---|---|---|---|
| |||
| Type 2 diabetes mellitus | Multifactorial | 3% of total population | Many |
| Type A receptor‐level insulin resistance | Congenital receptor defect | 86 cases | U‐500, insulin‐like growth factor‐1 |
| Type B receptor‐level insulin resistance | Antiinsulin receptor antibody | 50 cases | U‐500, immune modulation |
| Subcutaneous insulin resistance | Unknown; SQ protease? | 30 cases | U‐500, intraperitoneal insulin delivery, other |
Based on SQ insulin requirements, our patient had EIR. As his insulin levels failed to rise following an insulin injection, his EIR is thus subcutaneous in nature. However, among patients with this condition his failure to respond to IV insulin is unique. He does not fit criteria for types A or B insulin resistance; his condition is likely also due to an extreme version of the more common, multifactorial peripheral insulin resistance. This is supported by his successful response to the gastric bypass operation.5
The standard treatments for ESIR include: (1) concentrated regular insulin (U‐500) and (2) implantable intraperitoneal delivery; our patient received the former.6 U‐500 use in EIR has been shown to be more cost‐effective.1 Several reports have suggested success with protease inhibitors (aprotinin, nafamostat ointment), plasmapheresis, and intravenous immunoglobulin for extreme SQ resistance. Our case also represents the first treated successfully with a gastric bypass operation.
CONCLUSIONS
EIR can present a significant challenge for both the patient and hospitalist. The approach to this condition should begin with the determination of 24‐hour IV insulin requirement utilizing an insulin drip; serum insulin antibody evaluation; and endocrinology consultation. Our case also highlights a few important points about the broader management of diabetes mellitus. First, there are dermatological manifestations of diabetes that serve as potential markers for disease (namely acanthosis nigricans and NLD). Second, for patients with extreme insulin requirements, an extensive workup should be initiated and the patient should be transitioned to a concentrated regular insulin or intraperitoneal delivery. Third, our experience suggests a role for other measures such as gastric bypass that ought to be studied further.
A 34‐year‐old man was admitted for evaluation of elevated blood glucose despite extremely high subcutaneous (SQ) insulin requirements. He had a 12‐year history of Type 2 diabetes mellitus (T2DM) without episodes of ketoacidosis, managed initially with oral medications (metformin with various sulfonylureas and thiazolidinediones). Three months prior to admission, he was transitioned to SQ insulin and thereafter his requirements escalated rapidly. By the time of his admission, his blood glucose measurements were consistently above 300 mg/dL despite injecting more than 4100 units of insulin daily. His regimen included 300 units of insulin glargine (Lantus) 2 times per day (BID) and 1.75 mL of Humilin U‐500 Insulin (875 units) 4 times per day (QID). Past medical history included metabolic syndrome, nonalcoholic steatohepatitis, and diabetic neuropathy. Physical exam was remarkable for centripetal obesity (body mass index [BMI] = 38.9 kg/m2), acanthosis nigricans, and necrobiosis lipoidica diabeticorum (NLD) (Figure 1).

We undertook an investigation to characterize this extreme insulin resistance. After 24 hours without insulin supplementation, and 12 hours of nothing by mouth (NPO), his blood glucose level was 280 mg/dL and his serum insulin was 133.5 IU/mL. We injected 12 units of insulin Aspart and subsequently measured his serum glucose and insulin once more. His blood glucose level had risen to 289 mg/dL and his serum insulin fell to 110.7 IU/mL. We then transitioned the patient to intravenous (IV) insulin. After a series of boluses totaling 400 units, his blood glucose normalized (90 mg/dL) and was maintained in normal range on a rate of 48 units per hour. Over 24 hours, we had infused over 1400 units.
During this time, we also drew several labs. Serum antiinsulin antibodies were undetectable (ARUP Laboratories, Salt Lake City, UT). A full rheumatologic workup was negative for systemic lupus erythematosus (SLE), rheumatoid factor, Sjgren's syndrome (SS)‐A and SS‐B. Androgen levels were normal, as were 24‐hour urine collections for cortisol and metanephrines. The patient was discharged on a regimen of U‐500 without glargine.
By 5 months after discharge, his blood glucose remained uncontrolled despite increasing doses of U‐500 (with or without metformin and thiazolidinediones). The patient was offered a gastric bypass operation. Now, 4 months postoperative, his blood glucose is controlled, no greater than 90 mg/dL in the morning and 125 mg/dL in the evening. He is off insulin, taking 30 mg pioglitazone (Actos) daily and 500 mg metformin 3 times per day (TID).
Discussion
Extreme insulin resistance (EIR), defined by daily insulin requirements in excess of 200 U, is a rare and frustrating condition.1 Rarer still is extreme subcutaneous insulin resistance (ESIR). A systematic Medline review revealed only 29 reported cases of ESIR, all of which involved patients that maintained IV sensitivity to insulin. Classic diagnostic criteria for ESIR include preserved sensitivity to IV insulin, failure to increase serum insulin with subcutaneous injection, and insulin degrading activity of subcutaneous tissue.2, 3 However, there are, at present, no laboratory tests that can test the final criterion. Indeed, very few of the published reports of ESIR satisfy it, with most studies considering as diagnostic of ESIR the constellation of EIR with failure to raise serum insulin after injection and preserved intravenous insulin sensitivity.
As was evident in the high doses of IV insulin required for blood glucose normalization, our patient also had a proven receptor‐level peripheral resistance. Beyond the common, multifactorial insulin resistance of T2DM, the published reports of patients with extreme peripheral resistance are of 2 types: (A) genetic (eg, Leprechaunism) and (B) acquired autoimmune (Table 1).4 This patient fits neither category. Patients with Type A are very sick, with a syndromic disease that sharply curtails their life expectancy. Patients with Type B acquire antibodies directed against their insulin receptors and are almost invariably elderly African‐American women with severe rheumatological disease, namely SLE. We could not test our patient for an insulin‐receptor antibody secondary to prohibitive cost. This is probably moot, given that his autoimmune workup was negative and, as above, patients with such antibodies are vastly different compared to our patients.
| Class of Insulin Resistance | Mechanism | Incidence | Treatment |
|---|---|---|---|
| |||
| Type 2 diabetes mellitus | Multifactorial | 3% of total population | Many |
| Type A receptor‐level insulin resistance | Congenital receptor defect | 86 cases | U‐500, insulin‐like growth factor‐1 |
| Type B receptor‐level insulin resistance | Antiinsulin receptor antibody | 50 cases | U‐500, immune modulation |
| Subcutaneous insulin resistance | Unknown; SQ protease? | 30 cases | U‐500, intraperitoneal insulin delivery, other |
Based on SQ insulin requirements, our patient had EIR. As his insulin levels failed to rise following an insulin injection, his EIR is thus subcutaneous in nature. However, among patients with this condition his failure to respond to IV insulin is unique. He does not fit criteria for types A or B insulin resistance; his condition is likely also due to an extreme version of the more common, multifactorial peripheral insulin resistance. This is supported by his successful response to the gastric bypass operation.5
The standard treatments for ESIR include: (1) concentrated regular insulin (U‐500) and (2) implantable intraperitoneal delivery; our patient received the former.6 U‐500 use in EIR has been shown to be more cost‐effective.1 Several reports have suggested success with protease inhibitors (aprotinin, nafamostat ointment), plasmapheresis, and intravenous immunoglobulin for extreme SQ resistance. Our case also represents the first treated successfully with a gastric bypass operation.
CONCLUSIONS
EIR can present a significant challenge for both the patient and hospitalist. The approach to this condition should begin with the determination of 24‐hour IV insulin requirement utilizing an insulin drip; serum insulin antibody evaluation; and endocrinology consultation. Our case also highlights a few important points about the broader management of diabetes mellitus. First, there are dermatological manifestations of diabetes that serve as potential markers for disease (namely acanthosis nigricans and NLD). Second, for patients with extreme insulin requirements, an extensive workup should be initiated and the patient should be transitioned to a concentrated regular insulin or intraperitoneal delivery. Third, our experience suggests a role for other measures such as gastric bypass that ought to be studied further.
- ,,.The use of U‐500 in patients with extreme insulin resistance.Diabetes Care.2005;28:1240–1244.
- ,.Impaired absorption of insulin as a cause insulin resistance.Diabetes.1975;24:443.
- ,,.Insulin resistance caused by massive degradation of subcutaneous insulin.Diabetes.1979;28:640–645.
- ,,, et al.Clinical course of genetic diseases of the insulin receptor: a 30‐year prospective.Medicine.2004;83:209–222.
- ,,, et al.Who would have thought it? An operation proves to be the most effective therapy for adult‐onset diabetes mellitus.Ann Surg.1995;222:339–352.
- ,,,,,.Extreme subcutaneous insulin resistance: a misunderstood syndrome.Diabetes Metab.2003;29:539–546.
- ,,.The use of U‐500 in patients with extreme insulin resistance.Diabetes Care.2005;28:1240–1244.
- ,.Impaired absorption of insulin as a cause insulin resistance.Diabetes.1975;24:443.
- ,,.Insulin resistance caused by massive degradation of subcutaneous insulin.Diabetes.1979;28:640–645.
- ,,, et al.Clinical course of genetic diseases of the insulin receptor: a 30‐year prospective.Medicine.2004;83:209–222.
- ,,, et al.Who would have thought it? An operation proves to be the most effective therapy for adult‐onset diabetes mellitus.Ann Surg.1995;222:339–352.
- ,,,,,.Extreme subcutaneous insulin resistance: a misunderstood syndrome.Diabetes Metab.2003;29:539–546.