User login
The authors report no financial relationships relevant to this article.
Postmenopausal bleeding is a symptom evaluated often by general gynecologists. It necessitates assessment of the endometrium, most often by tissue sampling. When endometrial cancer is confirmed by biopsy, management becomes complex. Should the patient be referred to a gynecologic oncologist? What kind of surgery does she need? What kind of adjuvant treatment will be offered? Could the diagnosis be part of a genetic cancer syndrome?
Recent studies have yielded new information:
- Preoperative, intraoperative, and postoperative care by a gynecologic oncologist significantly lowers the cost of health care
- Lymphadenectomy for endometrial cancer remains controversial, and may be unnecessary in low-risk patients
- Chemotherapy plays an expanding role in the treatment of endometrial cancer. Adjuvant therapy with doxorubicin, cisplatin, and paclitaxel is the treatment of choice for patients who have advanced-stage disease
- Nine percent of women who are given a diagnosis of endometrial cancer before 50 years of age have a germ-line Lynch syndrome-associated mutation, which demonstrates that heredity is an important aspect of endometrial cancer and should be considered at all times.
It’s good economics to refer patients to gyn oncology sooner, not later
Hoekstra A, Singh DK, Garb M, Arekapudi S, Rademaker A, Lurain JR. Participation of the general gynecologist in the surgical staging of endometrial cancer: analysis of cost and perioperative outcomes. Gynecol Oncol. 2006;103:897–901.
Early-stage endometrial cancer is often curable with surgery alone because a full 88% of endometrial cancers present as clinical stage I.1 The role of the general gynecologist in surgical management of these cases is controversial; at some institutions, the practice is to call in the gynecologic oncologist for lymph-node sampling or when gross disease is identified; at others, the standard is to refer the patient to gynecologic oncology as soon as malignancy is diagnosed by endometrial biopsy. Hoekstra and colleagues have attempted to shed light on this issue with a retrospective chart review of 121 patients who were treated at one institution from 1998 to 2000.
Costs of early treatment by a gynecologic oncologist were lower than without referral
The authors performed a retrospective analysis of a group of women with clinical stage-I endometrial cancer who were treated surgically at Prentice Women’s Hospital in Chicago.
The cohort was divided in two:
- Group 1 comprised patients who underwent surgery with a general gynecologist, who consulted a gynecologic oncologist intraoperatively
- Group 2 comprised patients who were referred to a gynecologic oncologist before surgery and underwent the procedure with a gynecologic oncologist.
Overall, subjects in both groups were similar in age, distribution of surgical stage, need for lymphadenectomy, and length of follow-up.
Group 2 had a significantly shorter operative time overall, and shorter total time in the operating room. Cost per procedure was also significantly lower in this group, in terms of cost to the payer and the physician’s charge. Perioperative costs were also lower in Group 2.
No difference was observed in postoperative outcome. Total hospital costs and lengths of stay were also similar.
Recommendation for practice
With health-care costs rising, be aware of referral strategies that promote cost containment. Women who have endometrial cancer may benefit from the early involvement of a gynecologic oncologist.
Is lymphadenectomy necessary when risk of metastasis is low?
Mariani A, Dowdy S, Cliby W, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18.
The need for surgical staging of endometrial cancer has been recognized since surgical staging criteria were adopted by the International Federation of Gynecology and Obstetrics (FIGO) in 1988. Staging includes hysterectomy, bilateral salpingo-oophorectomy, and biopsy of any gross disease. Clear guidelines on the assessment of lymphatic dissemination and the anatomic extent of this assessment are, however, still lacking.
Proponents of systematic pelvic and para-aortic lymph-node dissection for patients with endometrial cancer cite:
- the 15% risk of lymph-node metastasis in women who have tumors 2 cm or larger in diameter2
- poor correlation between frozen-section grade and myometrial invasion with final pathology3
- the potential therapeutic benefit of the procedure.4
Opponents of such lymph-node dissection argue that women who have grade-1, stage-I disease will be overtreated if standardized lymphadenectomy is adopted.
Several retrospective studies have explored this question, with varying results. A large, prospective, randomized trial evaluating lymphadenectomy in clinical stage-I patients (ASTEC trial) has been completed, but is yet to be published.
When lymphadenectomy may (or may not) be necessary
After prospectively studying more than 300 endometrial cancer patients treated at the Mayo Clinic between 1984 and 1996,5 Mariani and colleagues launched a new study to assess a novel pattern of surgical management that aims to reduce the number of low-risk patients receiving lymphadenectomy. According to this pattern, the following types of women were able to bypass lymphadenectomy:
- those who had type-I, grades-1 and -2 tumors
- those with myometrial invasion ≤50%
- those with a primary tumor ≤2 cm in diameter.
Women who had endometrial cancer that did not meet these criteria underwent complete lymphadenectomy to the level of the renal vessels. Histologic assessment of the uterus to determine grade, depth of invasion, and primary tumor diameter was performed by frozen-section analysis in all cases.
The study included 422 women from January 2004 to December 2006. According to the guidelines of the study, 112 patients did not require lymphadenectomy. However, 22 (20%) women in this group did undergo the procedure because of palpable lymphadenopathy, initiation of dissection before the frozen-section report was received, or physician preference. All nodes were negative in these patients.
Of the women who met criteria for lymphadenectomy, 29 (9%) did not undergo dissection; among the reasons were disseminated disease, comorbid conditions, and advanced age. Of the women defined as at-risk who did undergo lymphadenectomy, 22% had lymph-node metastases.
Most positive para-aortic nodes lay above the inferior mesenteric artery
Information regarding the anatomic location of para-aortic nodal metastases was available for a small subset of women in the study. Seventy-seven percent of these women had para-aortic nodal metastasis above the inferior mesenteric artery. In addition, 71% of these patients had ipsilateral pelvic nodes that were free of disease. However, these patients had a poorer prognosis, and many would have received adjuvant therapy based on their hysterectomy specimen alone.
Recommendation for practice
This study suggests that there is a subset of patients who have endometrial cancer that is very low in risk and, because of this, they may forego lymph-node dissection without harm. In addition, a significant number of periaortic nodal metastases occur above the inferior mesenteric artery and in the absence of pelvic node involvement.
One of the limitations of this study is the need for intraoperative uterine assessment by frozen section by an expert pathologist—a service that is not widely available.
Taken together, these data suggest that, if the uterus can be assessed by frozen section at the time of surgery, a subset of clinical stage-I patients can be spared lymphadenectomy and its attendant risks.
Patients undergoing lymphatic assessment should undergo full systematic lymphnode dissection, not sampling. The dissection should include the region above the inferior mesenteric artery.
Removal of lymph nodes in endometrial cancer remains complex and controversial, a fact that strengthens the argument that an experienced gynecologic oncologist should be involved in the care of patients who have this disease.
Chemotherapy is warranted in advanced or recurrent disease
Homesley H, Filiaci V, Gibbons S, et al. Randomized phase III trial in advanced endometrial carcinoma of surgery and volume-directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Abstract presented at the Society of Gynecologic Oncologists, March 2008.
Adjuvant therapy for advanced-stage endometrial cancer has varied considerably over the years, and treatment of these patients remains somewhat controversial. Clinical trials comparing chemotherapy with radiation, and chemotherapy regimens with each other, have led to an era in which chemotherapy is used to treat more women than ever before.
In 2006, the Gynecologic Oncology Group (GOG) published the results of a prospective randomized study (GOG 122) that compared whole-abdomen radiation to doxorubicin and cisplatin in stage-III or -IV endometrial cancer. The investigators determined that chemotherapy was superior to whole-abdomen radiation in this trial.6
This finding was quickly followed by another trial (GOG 177) in which doxorubicin plus cisplatin was compared with a regimen of doxorubicin, cisplatin, and paclitaxel. Women in this trial had stage-III or -IV or recurrent disease. A history of radiation treatment did not disqualify patients from the study, and the treatment groups were well balanced in randomization. The doxorubicin–cisplatin–paclitaxel arm improved progression-free and overall survival, making this combination the preferred treatment.7
After volume-directed radiation, paclitaxel does not add benefit
Several retrospective analyses of radiation versus chemotherapy have shown improvement with radiation or combination therapy.8 Most recently, the GOG released data from a trial comparing chemotherapy regimens after radiation treatment. GOG 184 evaluated surgically debulked, stage-III or -IV patients who had received volume-directed radiation; subjects were randomized into either of two chemotherapy regimens:
- doxorubicin plus cisplatin
- doxorubicin, cisplatin, and paclitaxel.
In these patients, there was no improvement in survival when paclitaxel was added to the chemotherapeutic regimen, compared with doxorubicin plus cisplatin. Morbidity increased, however, with the addition of paclitaxel.
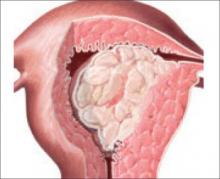
Patients in this trial underwent surgical resection of all gross disease, with no residual tumor larger than 2 cm. The role of optimal cytoreduction in endometrial cancer has been debated, however. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter (FIGURE).9-11 GOG 184 inclusion criteria required surgical resection of gross disease to ≤2 cm in diameter. It is possible that the therapeutic benefit of surgical debulking may have improved outcome in these patients—to the extent that the addition of paclitaxel did not provide appreciable benefit.
FIGURE Debulk gross disease?
The role of optimal cytoreduction in endometrial cancer has been debated. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter.
Recommendation for practice
Data on adjuvant therapy for endometrial cancer remains conflicting. Women who have advanced-stage or recurrent endometrial cancer should receive chemotherapy—either with doxorubicin, cisplatin, and paclitaxel, or a platinum taxane regimen. The addition of paclitaxel in surgically debulked patients who have undergone radiation treatment does not appear to improve survival.
There is, however, a clear recommendation for paclitaxel in radiation-naïve patients and those who have gross residual disease. Further studies are needed to elucidate the role of radiation therapy in an era of volume-directed radiation.
Look for Lynch syndrome in young women with endometrial cancer
Lu K, Schorge J, Rodabaugh K, et al. Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164.
Endometrial cancer is part of the spectrum of Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome). Patients with this autosomal-dominant hereditary cancer susceptibility syndrome may present with colorectal cancer, endometrial cancer, or, more rarely, ovarian cancer. Lynch syndrome derives from germline mutations in DNA mismatch repair genes, most often MLH1, MSH2, and MSH6.12 Genetic testing for all three genes is available for clinical use.
In the past, screening for Lynch syndrome focused on colorectal cancer, but it is now clear that women who have this disorder have a lifetime risk of developing endometrial cancer that exceeds 40%.13
In Lynch syndrome, gynecologic cancer often precedes colon cancer
Women with Lynch syndrome-associated endometrial cancer typically present at a younger age than their syndrome-free counterparts (48 compared with 60 years).14 Previous retrospective studies demonstrated that 50% of women with Lynch syndrome-associated colon and gynecologic cancers had gynecologic cancer preceding the colon cancer. The average was 11 years earlier for endometrial cancer, which suggests that, if these women could be identified at the time they are given their diagnosis of endometrial cancer, more intensive screening for colon cancer could then be initiated.15
9% of women who develop endometrial cancer before age 50 have Lynch syndrome
One of the screening criteria for Lynch syndrome-associated colon cancer is age <50 years. In this recent prospective, multicenter study involving 100 women who were diagnosed with endometrial cancer at less than 50 years of age, germline Lynch syndrome mutations were identified in 9% of patients. (In this study, germline mutation testing was performed for MLH1, MSH2, and MSH6 genes by full sequencing, and immunohistochemistry was performed for all three genes. Microsatellite analysis was performed on 95 patients, with five women having insufficient tumor for DNA extraction.)
All women who had a germline mutation had a first-degree relative with Lynch syndrome-associated cancer. The combination of a negative family history for Lynch syndrome and a body mass index greater than 30 was highly predictive of having no Lynch syndrome mutation, with a negative predictive value of 96%.
Recommendation for practice
Patients who have an hereditary cancer syndrome such as Lynch syndrome can begin cancer-prevention screening—and be engaged in that screening—when the syndrome is recognized early. Because women who have endometrial cancer that was diagnosed before they were 50 years old are at significant risk of a germline mutation, they should be offered genetic counseling and testing.
1. Fanning J, Hoffman ML, Andrews SJ, Harrah AW, Feldmeier JJ. Cost-effectiveness analysis of the treatment for intermediate risk endometrial cancer: postoperative brachytherapy vs. observation. Gynecol Oncol. 2004;93:632-636.
2. Schink JC, Rademaker AW, Miller DS, Lurain JR. Tumor size in endometrial cancer. Cancer. 1991;67:2791-2794.
3. Case AS, Rocconi RP, Straughn JM Jr, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006;108:1375-1379.
4. Horowitz NS, Peters WA, 3rd, Smith MR, Drescher CW, Atwood M, Mate TP. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet Gynecol. 2002;99:235-240.
5. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506-1519.
6. Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36-44.
7. Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159-2166.
8. Alvarez Secord A, Havrilesky LJ, Bac-Jump V, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007;107:285-291.
9. Bristow RE, Zerbe MJ, Rosenshein NB, Grumbine FC, Montz FJ. Stage IVB endometrial carcinoma: the role of cytoreductive surgery and determinants of survival. Gynecol Oncol. 2000;78:85-91.
10. Bristow RE, Santillan A, Zahurak ML, Gardner GJ, Giuntoli RL, 2nd, Armstrong DK. Salvage cytoreductive surgery for recurrent endometrial cancer. Gynecol Oncol. 2006;103:281-287.
11. Lambrou NC, Gómez-Marín O, Mirhashemi R, et al. Optimal surgical cytoreduction in patients with Stage III and Stage IV endometrial carcinoma: a study of morbidity and survival. Gynecol Oncol. 2004;93:653-658.
12. Peltomaki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborate study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997;113:1146-1158.
13. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
14. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on HNPCC. Anticancer Res. 1994;14:1661-1664.
15. Lu KH, Broaddus RR. Gynecological tumors in hereditary nonpolyposis colorectal cancer: we know they are common—now what? Gynecol Oncol. 2001;82:221-222.
The authors report no financial relationships relevant to this article.
Postmenopausal bleeding is a symptom evaluated often by general gynecologists. It necessitates assessment of the endometrium, most often by tissue sampling. When endometrial cancer is confirmed by biopsy, management becomes complex. Should the patient be referred to a gynecologic oncologist? What kind of surgery does she need? What kind of adjuvant treatment will be offered? Could the diagnosis be part of a genetic cancer syndrome?
Recent studies have yielded new information:
- Preoperative, intraoperative, and postoperative care by a gynecologic oncologist significantly lowers the cost of health care
- Lymphadenectomy for endometrial cancer remains controversial, and may be unnecessary in low-risk patients
- Chemotherapy plays an expanding role in the treatment of endometrial cancer. Adjuvant therapy with doxorubicin, cisplatin, and paclitaxel is the treatment of choice for patients who have advanced-stage disease
- Nine percent of women who are given a diagnosis of endometrial cancer before 50 years of age have a germ-line Lynch syndrome-associated mutation, which demonstrates that heredity is an important aspect of endometrial cancer and should be considered at all times.
It’s good economics to refer patients to gyn oncology sooner, not later
Hoekstra A, Singh DK, Garb M, Arekapudi S, Rademaker A, Lurain JR. Participation of the general gynecologist in the surgical staging of endometrial cancer: analysis of cost and perioperative outcomes. Gynecol Oncol. 2006;103:897–901.
Early-stage endometrial cancer is often curable with surgery alone because a full 88% of endometrial cancers present as clinical stage I.1 The role of the general gynecologist in surgical management of these cases is controversial; at some institutions, the practice is to call in the gynecologic oncologist for lymph-node sampling or when gross disease is identified; at others, the standard is to refer the patient to gynecologic oncology as soon as malignancy is diagnosed by endometrial biopsy. Hoekstra and colleagues have attempted to shed light on this issue with a retrospective chart review of 121 patients who were treated at one institution from 1998 to 2000.
Costs of early treatment by a gynecologic oncologist were lower than without referral
The authors performed a retrospective analysis of a group of women with clinical stage-I endometrial cancer who were treated surgically at Prentice Women’s Hospital in Chicago.
The cohort was divided in two:
- Group 1 comprised patients who underwent surgery with a general gynecologist, who consulted a gynecologic oncologist intraoperatively
- Group 2 comprised patients who were referred to a gynecologic oncologist before surgery and underwent the procedure with a gynecologic oncologist.
Overall, subjects in both groups were similar in age, distribution of surgical stage, need for lymphadenectomy, and length of follow-up.
Group 2 had a significantly shorter operative time overall, and shorter total time in the operating room. Cost per procedure was also significantly lower in this group, in terms of cost to the payer and the physician’s charge. Perioperative costs were also lower in Group 2.
No difference was observed in postoperative outcome. Total hospital costs and lengths of stay were also similar.
Recommendation for practice
With health-care costs rising, be aware of referral strategies that promote cost containment. Women who have endometrial cancer may benefit from the early involvement of a gynecologic oncologist.
Is lymphadenectomy necessary when risk of metastasis is low?
Mariani A, Dowdy S, Cliby W, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18.
The need for surgical staging of endometrial cancer has been recognized since surgical staging criteria were adopted by the International Federation of Gynecology and Obstetrics (FIGO) in 1988. Staging includes hysterectomy, bilateral salpingo-oophorectomy, and biopsy of any gross disease. Clear guidelines on the assessment of lymphatic dissemination and the anatomic extent of this assessment are, however, still lacking.
Proponents of systematic pelvic and para-aortic lymph-node dissection for patients with endometrial cancer cite:
- the 15% risk of lymph-node metastasis in women who have tumors 2 cm or larger in diameter2
- poor correlation between frozen-section grade and myometrial invasion with final pathology3
- the potential therapeutic benefit of the procedure.4
Opponents of such lymph-node dissection argue that women who have grade-1, stage-I disease will be overtreated if standardized lymphadenectomy is adopted.
Several retrospective studies have explored this question, with varying results. A large, prospective, randomized trial evaluating lymphadenectomy in clinical stage-I patients (ASTEC trial) has been completed, but is yet to be published.
When lymphadenectomy may (or may not) be necessary
After prospectively studying more than 300 endometrial cancer patients treated at the Mayo Clinic between 1984 and 1996,5 Mariani and colleagues launched a new study to assess a novel pattern of surgical management that aims to reduce the number of low-risk patients receiving lymphadenectomy. According to this pattern, the following types of women were able to bypass lymphadenectomy:
- those who had type-I, grades-1 and -2 tumors
- those with myometrial invasion ≤50%
- those with a primary tumor ≤2 cm in diameter.
Women who had endometrial cancer that did not meet these criteria underwent complete lymphadenectomy to the level of the renal vessels. Histologic assessment of the uterus to determine grade, depth of invasion, and primary tumor diameter was performed by frozen-section analysis in all cases.
The study included 422 women from January 2004 to December 2006. According to the guidelines of the study, 112 patients did not require lymphadenectomy. However, 22 (20%) women in this group did undergo the procedure because of palpable lymphadenopathy, initiation of dissection before the frozen-section report was received, or physician preference. All nodes were negative in these patients.
Of the women who met criteria for lymphadenectomy, 29 (9%) did not undergo dissection; among the reasons were disseminated disease, comorbid conditions, and advanced age. Of the women defined as at-risk who did undergo lymphadenectomy, 22% had lymph-node metastases.
Most positive para-aortic nodes lay above the inferior mesenteric artery
Information regarding the anatomic location of para-aortic nodal metastases was available for a small subset of women in the study. Seventy-seven percent of these women had para-aortic nodal metastasis above the inferior mesenteric artery. In addition, 71% of these patients had ipsilateral pelvic nodes that were free of disease. However, these patients had a poorer prognosis, and many would have received adjuvant therapy based on their hysterectomy specimen alone.
Recommendation for practice
This study suggests that there is a subset of patients who have endometrial cancer that is very low in risk and, because of this, they may forego lymph-node dissection without harm. In addition, a significant number of periaortic nodal metastases occur above the inferior mesenteric artery and in the absence of pelvic node involvement.
One of the limitations of this study is the need for intraoperative uterine assessment by frozen section by an expert pathologist—a service that is not widely available.
Taken together, these data suggest that, if the uterus can be assessed by frozen section at the time of surgery, a subset of clinical stage-I patients can be spared lymphadenectomy and its attendant risks.
Patients undergoing lymphatic assessment should undergo full systematic lymphnode dissection, not sampling. The dissection should include the region above the inferior mesenteric artery.
Removal of lymph nodes in endometrial cancer remains complex and controversial, a fact that strengthens the argument that an experienced gynecologic oncologist should be involved in the care of patients who have this disease.
Chemotherapy is warranted in advanced or recurrent disease
Homesley H, Filiaci V, Gibbons S, et al. Randomized phase III trial in advanced endometrial carcinoma of surgery and volume-directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Abstract presented at the Society of Gynecologic Oncologists, March 2008.
Adjuvant therapy for advanced-stage endometrial cancer has varied considerably over the years, and treatment of these patients remains somewhat controversial. Clinical trials comparing chemotherapy with radiation, and chemotherapy regimens with each other, have led to an era in which chemotherapy is used to treat more women than ever before.
In 2006, the Gynecologic Oncology Group (GOG) published the results of a prospective randomized study (GOG 122) that compared whole-abdomen radiation to doxorubicin and cisplatin in stage-III or -IV endometrial cancer. The investigators determined that chemotherapy was superior to whole-abdomen radiation in this trial.6
This finding was quickly followed by another trial (GOG 177) in which doxorubicin plus cisplatin was compared with a regimen of doxorubicin, cisplatin, and paclitaxel. Women in this trial had stage-III or -IV or recurrent disease. A history of radiation treatment did not disqualify patients from the study, and the treatment groups were well balanced in randomization. The doxorubicin–cisplatin–paclitaxel arm improved progression-free and overall survival, making this combination the preferred treatment.7
After volume-directed radiation, paclitaxel does not add benefit
Several retrospective analyses of radiation versus chemotherapy have shown improvement with radiation or combination therapy.8 Most recently, the GOG released data from a trial comparing chemotherapy regimens after radiation treatment. GOG 184 evaluated surgically debulked, stage-III or -IV patients who had received volume-directed radiation; subjects were randomized into either of two chemotherapy regimens:
- doxorubicin plus cisplatin
- doxorubicin, cisplatin, and paclitaxel.
In these patients, there was no improvement in survival when paclitaxel was added to the chemotherapeutic regimen, compared with doxorubicin plus cisplatin. Morbidity increased, however, with the addition of paclitaxel.
Patients in this trial underwent surgical resection of all gross disease, with no residual tumor larger than 2 cm. The role of optimal cytoreduction in endometrial cancer has been debated, however. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter (FIGURE).9-11 GOG 184 inclusion criteria required surgical resection of gross disease to ≤2 cm in diameter. It is possible that the therapeutic benefit of surgical debulking may have improved outcome in these patients—to the extent that the addition of paclitaxel did not provide appreciable benefit.
FIGURE Debulk gross disease?
The role of optimal cytoreduction in endometrial cancer has been debated. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter.
Recommendation for practice
Data on adjuvant therapy for endometrial cancer remains conflicting. Women who have advanced-stage or recurrent endometrial cancer should receive chemotherapy—either with doxorubicin, cisplatin, and paclitaxel, or a platinum taxane regimen. The addition of paclitaxel in surgically debulked patients who have undergone radiation treatment does not appear to improve survival.
There is, however, a clear recommendation for paclitaxel in radiation-naïve patients and those who have gross residual disease. Further studies are needed to elucidate the role of radiation therapy in an era of volume-directed radiation.
Look for Lynch syndrome in young women with endometrial cancer
Lu K, Schorge J, Rodabaugh K, et al. Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164.
Endometrial cancer is part of the spectrum of Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome). Patients with this autosomal-dominant hereditary cancer susceptibility syndrome may present with colorectal cancer, endometrial cancer, or, more rarely, ovarian cancer. Lynch syndrome derives from germline mutations in DNA mismatch repair genes, most often MLH1, MSH2, and MSH6.12 Genetic testing for all three genes is available for clinical use.
In the past, screening for Lynch syndrome focused on colorectal cancer, but it is now clear that women who have this disorder have a lifetime risk of developing endometrial cancer that exceeds 40%.13
In Lynch syndrome, gynecologic cancer often precedes colon cancer
Women with Lynch syndrome-associated endometrial cancer typically present at a younger age than their syndrome-free counterparts (48 compared with 60 years).14 Previous retrospective studies demonstrated that 50% of women with Lynch syndrome-associated colon and gynecologic cancers had gynecologic cancer preceding the colon cancer. The average was 11 years earlier for endometrial cancer, which suggests that, if these women could be identified at the time they are given their diagnosis of endometrial cancer, more intensive screening for colon cancer could then be initiated.15
9% of women who develop endometrial cancer before age 50 have Lynch syndrome
One of the screening criteria for Lynch syndrome-associated colon cancer is age <50 years. In this recent prospective, multicenter study involving 100 women who were diagnosed with endometrial cancer at less than 50 years of age, germline Lynch syndrome mutations were identified in 9% of patients. (In this study, germline mutation testing was performed for MLH1, MSH2, and MSH6 genes by full sequencing, and immunohistochemistry was performed for all three genes. Microsatellite analysis was performed on 95 patients, with five women having insufficient tumor for DNA extraction.)
All women who had a germline mutation had a first-degree relative with Lynch syndrome-associated cancer. The combination of a negative family history for Lynch syndrome and a body mass index greater than 30 was highly predictive of having no Lynch syndrome mutation, with a negative predictive value of 96%.
Recommendation for practice
Patients who have an hereditary cancer syndrome such as Lynch syndrome can begin cancer-prevention screening—and be engaged in that screening—when the syndrome is recognized early. Because women who have endometrial cancer that was diagnosed before they were 50 years old are at significant risk of a germline mutation, they should be offered genetic counseling and testing.
The authors report no financial relationships relevant to this article.
Postmenopausal bleeding is a symptom evaluated often by general gynecologists. It necessitates assessment of the endometrium, most often by tissue sampling. When endometrial cancer is confirmed by biopsy, management becomes complex. Should the patient be referred to a gynecologic oncologist? What kind of surgery does she need? What kind of adjuvant treatment will be offered? Could the diagnosis be part of a genetic cancer syndrome?
Recent studies have yielded new information:
- Preoperative, intraoperative, and postoperative care by a gynecologic oncologist significantly lowers the cost of health care
- Lymphadenectomy for endometrial cancer remains controversial, and may be unnecessary in low-risk patients
- Chemotherapy plays an expanding role in the treatment of endometrial cancer. Adjuvant therapy with doxorubicin, cisplatin, and paclitaxel is the treatment of choice for patients who have advanced-stage disease
- Nine percent of women who are given a diagnosis of endometrial cancer before 50 years of age have a germ-line Lynch syndrome-associated mutation, which demonstrates that heredity is an important aspect of endometrial cancer and should be considered at all times.
It’s good economics to refer patients to gyn oncology sooner, not later
Hoekstra A, Singh DK, Garb M, Arekapudi S, Rademaker A, Lurain JR. Participation of the general gynecologist in the surgical staging of endometrial cancer: analysis of cost and perioperative outcomes. Gynecol Oncol. 2006;103:897–901.
Early-stage endometrial cancer is often curable with surgery alone because a full 88% of endometrial cancers present as clinical stage I.1 The role of the general gynecologist in surgical management of these cases is controversial; at some institutions, the practice is to call in the gynecologic oncologist for lymph-node sampling or when gross disease is identified; at others, the standard is to refer the patient to gynecologic oncology as soon as malignancy is diagnosed by endometrial biopsy. Hoekstra and colleagues have attempted to shed light on this issue with a retrospective chart review of 121 patients who were treated at one institution from 1998 to 2000.
Costs of early treatment by a gynecologic oncologist were lower than without referral
The authors performed a retrospective analysis of a group of women with clinical stage-I endometrial cancer who were treated surgically at Prentice Women’s Hospital in Chicago.
The cohort was divided in two:
- Group 1 comprised patients who underwent surgery with a general gynecologist, who consulted a gynecologic oncologist intraoperatively
- Group 2 comprised patients who were referred to a gynecologic oncologist before surgery and underwent the procedure with a gynecologic oncologist.
Overall, subjects in both groups were similar in age, distribution of surgical stage, need for lymphadenectomy, and length of follow-up.
Group 2 had a significantly shorter operative time overall, and shorter total time in the operating room. Cost per procedure was also significantly lower in this group, in terms of cost to the payer and the physician’s charge. Perioperative costs were also lower in Group 2.
No difference was observed in postoperative outcome. Total hospital costs and lengths of stay were also similar.
Recommendation for practice
With health-care costs rising, be aware of referral strategies that promote cost containment. Women who have endometrial cancer may benefit from the early involvement of a gynecologic oncologist.
Is lymphadenectomy necessary when risk of metastasis is low?
Mariani A, Dowdy S, Cliby W, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18.
The need for surgical staging of endometrial cancer has been recognized since surgical staging criteria were adopted by the International Federation of Gynecology and Obstetrics (FIGO) in 1988. Staging includes hysterectomy, bilateral salpingo-oophorectomy, and biopsy of any gross disease. Clear guidelines on the assessment of lymphatic dissemination and the anatomic extent of this assessment are, however, still lacking.
Proponents of systematic pelvic and para-aortic lymph-node dissection for patients with endometrial cancer cite:
- the 15% risk of lymph-node metastasis in women who have tumors 2 cm or larger in diameter2
- poor correlation between frozen-section grade and myometrial invasion with final pathology3
- the potential therapeutic benefit of the procedure.4
Opponents of such lymph-node dissection argue that women who have grade-1, stage-I disease will be overtreated if standardized lymphadenectomy is adopted.
Several retrospective studies have explored this question, with varying results. A large, prospective, randomized trial evaluating lymphadenectomy in clinical stage-I patients (ASTEC trial) has been completed, but is yet to be published.
When lymphadenectomy may (or may not) be necessary
After prospectively studying more than 300 endometrial cancer patients treated at the Mayo Clinic between 1984 and 1996,5 Mariani and colleagues launched a new study to assess a novel pattern of surgical management that aims to reduce the number of low-risk patients receiving lymphadenectomy. According to this pattern, the following types of women were able to bypass lymphadenectomy:
- those who had type-I, grades-1 and -2 tumors
- those with myometrial invasion ≤50%
- those with a primary tumor ≤2 cm in diameter.
Women who had endometrial cancer that did not meet these criteria underwent complete lymphadenectomy to the level of the renal vessels. Histologic assessment of the uterus to determine grade, depth of invasion, and primary tumor diameter was performed by frozen-section analysis in all cases.
The study included 422 women from January 2004 to December 2006. According to the guidelines of the study, 112 patients did not require lymphadenectomy. However, 22 (20%) women in this group did undergo the procedure because of palpable lymphadenopathy, initiation of dissection before the frozen-section report was received, or physician preference. All nodes were negative in these patients.
Of the women who met criteria for lymphadenectomy, 29 (9%) did not undergo dissection; among the reasons were disseminated disease, comorbid conditions, and advanced age. Of the women defined as at-risk who did undergo lymphadenectomy, 22% had lymph-node metastases.
Most positive para-aortic nodes lay above the inferior mesenteric artery
Information regarding the anatomic location of para-aortic nodal metastases was available for a small subset of women in the study. Seventy-seven percent of these women had para-aortic nodal metastasis above the inferior mesenteric artery. In addition, 71% of these patients had ipsilateral pelvic nodes that were free of disease. However, these patients had a poorer prognosis, and many would have received adjuvant therapy based on their hysterectomy specimen alone.
Recommendation for practice
This study suggests that there is a subset of patients who have endometrial cancer that is very low in risk and, because of this, they may forego lymph-node dissection without harm. In addition, a significant number of periaortic nodal metastases occur above the inferior mesenteric artery and in the absence of pelvic node involvement.
One of the limitations of this study is the need for intraoperative uterine assessment by frozen section by an expert pathologist—a service that is not widely available.
Taken together, these data suggest that, if the uterus can be assessed by frozen section at the time of surgery, a subset of clinical stage-I patients can be spared lymphadenectomy and its attendant risks.
Patients undergoing lymphatic assessment should undergo full systematic lymphnode dissection, not sampling. The dissection should include the region above the inferior mesenteric artery.
Removal of lymph nodes in endometrial cancer remains complex and controversial, a fact that strengthens the argument that an experienced gynecologic oncologist should be involved in the care of patients who have this disease.
Chemotherapy is warranted in advanced or recurrent disease
Homesley H, Filiaci V, Gibbons S, et al. Randomized phase III trial in advanced endometrial carcinoma of surgery and volume-directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Abstract presented at the Society of Gynecologic Oncologists, March 2008.
Adjuvant therapy for advanced-stage endometrial cancer has varied considerably over the years, and treatment of these patients remains somewhat controversial. Clinical trials comparing chemotherapy with radiation, and chemotherapy regimens with each other, have led to an era in which chemotherapy is used to treat more women than ever before.
In 2006, the Gynecologic Oncology Group (GOG) published the results of a prospective randomized study (GOG 122) that compared whole-abdomen radiation to doxorubicin and cisplatin in stage-III or -IV endometrial cancer. The investigators determined that chemotherapy was superior to whole-abdomen radiation in this trial.6
This finding was quickly followed by another trial (GOG 177) in which doxorubicin plus cisplatin was compared with a regimen of doxorubicin, cisplatin, and paclitaxel. Women in this trial had stage-III or -IV or recurrent disease. A history of radiation treatment did not disqualify patients from the study, and the treatment groups were well balanced in randomization. The doxorubicin–cisplatin–paclitaxel arm improved progression-free and overall survival, making this combination the preferred treatment.7
After volume-directed radiation, paclitaxel does not add benefit
Several retrospective analyses of radiation versus chemotherapy have shown improvement with radiation or combination therapy.8 Most recently, the GOG released data from a trial comparing chemotherapy regimens after radiation treatment. GOG 184 evaluated surgically debulked, stage-III or -IV patients who had received volume-directed radiation; subjects were randomized into either of two chemotherapy regimens:
- doxorubicin plus cisplatin
- doxorubicin, cisplatin, and paclitaxel.
In these patients, there was no improvement in survival when paclitaxel was added to the chemotherapeutic regimen, compared with doxorubicin plus cisplatin. Morbidity increased, however, with the addition of paclitaxel.
Patients in this trial underwent surgical resection of all gross disease, with no residual tumor larger than 2 cm. The role of optimal cytoreduction in endometrial cancer has been debated, however. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter (FIGURE).9-11 GOG 184 inclusion criteria required surgical resection of gross disease to ≤2 cm in diameter. It is possible that the therapeutic benefit of surgical debulking may have improved outcome in these patients—to the extent that the addition of paclitaxel did not provide appreciable benefit.
FIGURE Debulk gross disease?
The role of optimal cytoreduction in endometrial cancer has been debated. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter.
Recommendation for practice
Data on adjuvant therapy for endometrial cancer remains conflicting. Women who have advanced-stage or recurrent endometrial cancer should receive chemotherapy—either with doxorubicin, cisplatin, and paclitaxel, or a platinum taxane regimen. The addition of paclitaxel in surgically debulked patients who have undergone radiation treatment does not appear to improve survival.
There is, however, a clear recommendation for paclitaxel in radiation-naïve patients and those who have gross residual disease. Further studies are needed to elucidate the role of radiation therapy in an era of volume-directed radiation.
Look for Lynch syndrome in young women with endometrial cancer
Lu K, Schorge J, Rodabaugh K, et al. Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164.
Endometrial cancer is part of the spectrum of Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome). Patients with this autosomal-dominant hereditary cancer susceptibility syndrome may present with colorectal cancer, endometrial cancer, or, more rarely, ovarian cancer. Lynch syndrome derives from germline mutations in DNA mismatch repair genes, most often MLH1, MSH2, and MSH6.12 Genetic testing for all three genes is available for clinical use.
In the past, screening for Lynch syndrome focused on colorectal cancer, but it is now clear that women who have this disorder have a lifetime risk of developing endometrial cancer that exceeds 40%.13
In Lynch syndrome, gynecologic cancer often precedes colon cancer
Women with Lynch syndrome-associated endometrial cancer typically present at a younger age than their syndrome-free counterparts (48 compared with 60 years).14 Previous retrospective studies demonstrated that 50% of women with Lynch syndrome-associated colon and gynecologic cancers had gynecologic cancer preceding the colon cancer. The average was 11 years earlier for endometrial cancer, which suggests that, if these women could be identified at the time they are given their diagnosis of endometrial cancer, more intensive screening for colon cancer could then be initiated.15
9% of women who develop endometrial cancer before age 50 have Lynch syndrome
One of the screening criteria for Lynch syndrome-associated colon cancer is age <50 years. In this recent prospective, multicenter study involving 100 women who were diagnosed with endometrial cancer at less than 50 years of age, germline Lynch syndrome mutations were identified in 9% of patients. (In this study, germline mutation testing was performed for MLH1, MSH2, and MSH6 genes by full sequencing, and immunohistochemistry was performed for all three genes. Microsatellite analysis was performed on 95 patients, with five women having insufficient tumor for DNA extraction.)
All women who had a germline mutation had a first-degree relative with Lynch syndrome-associated cancer. The combination of a negative family history for Lynch syndrome and a body mass index greater than 30 was highly predictive of having no Lynch syndrome mutation, with a negative predictive value of 96%.
Recommendation for practice
Patients who have an hereditary cancer syndrome such as Lynch syndrome can begin cancer-prevention screening—and be engaged in that screening—when the syndrome is recognized early. Because women who have endometrial cancer that was diagnosed before they were 50 years old are at significant risk of a germline mutation, they should be offered genetic counseling and testing.
1. Fanning J, Hoffman ML, Andrews SJ, Harrah AW, Feldmeier JJ. Cost-effectiveness analysis of the treatment for intermediate risk endometrial cancer: postoperative brachytherapy vs. observation. Gynecol Oncol. 2004;93:632-636.
2. Schink JC, Rademaker AW, Miller DS, Lurain JR. Tumor size in endometrial cancer. Cancer. 1991;67:2791-2794.
3. Case AS, Rocconi RP, Straughn JM Jr, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006;108:1375-1379.
4. Horowitz NS, Peters WA, 3rd, Smith MR, Drescher CW, Atwood M, Mate TP. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet Gynecol. 2002;99:235-240.
5. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506-1519.
6. Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36-44.
7. Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159-2166.
8. Alvarez Secord A, Havrilesky LJ, Bac-Jump V, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007;107:285-291.
9. Bristow RE, Zerbe MJ, Rosenshein NB, Grumbine FC, Montz FJ. Stage IVB endometrial carcinoma: the role of cytoreductive surgery and determinants of survival. Gynecol Oncol. 2000;78:85-91.
10. Bristow RE, Santillan A, Zahurak ML, Gardner GJ, Giuntoli RL, 2nd, Armstrong DK. Salvage cytoreductive surgery for recurrent endometrial cancer. Gynecol Oncol. 2006;103:281-287.
11. Lambrou NC, Gómez-Marín O, Mirhashemi R, et al. Optimal surgical cytoreduction in patients with Stage III and Stage IV endometrial carcinoma: a study of morbidity and survival. Gynecol Oncol. 2004;93:653-658.
12. Peltomaki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborate study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997;113:1146-1158.
13. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
14. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on HNPCC. Anticancer Res. 1994;14:1661-1664.
15. Lu KH, Broaddus RR. Gynecological tumors in hereditary nonpolyposis colorectal cancer: we know they are common—now what? Gynecol Oncol. 2001;82:221-222.
1. Fanning J, Hoffman ML, Andrews SJ, Harrah AW, Feldmeier JJ. Cost-effectiveness analysis of the treatment for intermediate risk endometrial cancer: postoperative brachytherapy vs. observation. Gynecol Oncol. 2004;93:632-636.
2. Schink JC, Rademaker AW, Miller DS, Lurain JR. Tumor size in endometrial cancer. Cancer. 1991;67:2791-2794.
3. Case AS, Rocconi RP, Straughn JM Jr, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006;108:1375-1379.
4. Horowitz NS, Peters WA, 3rd, Smith MR, Drescher CW, Atwood M, Mate TP. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet Gynecol. 2002;99:235-240.
5. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506-1519.
6. Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36-44.
7. Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159-2166.
8. Alvarez Secord A, Havrilesky LJ, Bac-Jump V, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007;107:285-291.
9. Bristow RE, Zerbe MJ, Rosenshein NB, Grumbine FC, Montz FJ. Stage IVB endometrial carcinoma: the role of cytoreductive surgery and determinants of survival. Gynecol Oncol. 2000;78:85-91.
10. Bristow RE, Santillan A, Zahurak ML, Gardner GJ, Giuntoli RL, 2nd, Armstrong DK. Salvage cytoreductive surgery for recurrent endometrial cancer. Gynecol Oncol. 2006;103:281-287.
11. Lambrou NC, Gómez-Marín O, Mirhashemi R, et al. Optimal surgical cytoreduction in patients with Stage III and Stage IV endometrial carcinoma: a study of morbidity and survival. Gynecol Oncol. 2004;93:653-658.
12. Peltomaki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborate study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997;113:1146-1158.
13. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
14. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on HNPCC. Anticancer Res. 1994;14:1661-1664.
15. Lu KH, Broaddus RR. Gynecological tumors in hereditary nonpolyposis colorectal cancer: we know they are common—now what? Gynecol Oncol. 2001;82:221-222.