User login
CHICAGO – Twenty-four weeks of treatment with empagliflozin in patients with type 2 diabetes achieved clinically meaningful improvements in cardiovascular risk factors as well as in glycemic control, according to pooled data from four pivotal phase III clinical trials.
Empagliflozin is a sodium glucose cotransporter 2 (SGLT2) inhibitor. This is a novel emerging class of oral diabetic medications that work by boosting urinary glucose excretion. Canagliflozin (Invokana), the first-in-class SGLT2 inhibitor, received Food and Drug Administration marketing approval in March for treatment of type 2 diabetes. Empagliflozin’s application for marketing approval is now under review by the FDA and the European Medicines Agency, Dr. Thomas Hach noted at the annual scientific sessions of the American Diabetes Association.
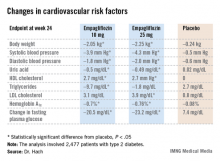
Empagliflozin resulted in significant, clinically meaningful improvements in blood pressure, body weight, uric acid, HDL cholesterol, hemoglobin A1c, and fasting plasma glucose, along with a modest, albeit unwelcome, increase in LDL cholesterol.
The improvement in blood pressure was particularly noteworthy. Among patients with uncontrolled blood pressure at baseline – that is, blood pressures of 130/80 mm Hg or higher – 33.3% of those who received empagliflozin at 10 mg/day and 32.2% on empagliflozin at 25 mg/day had controlled blood pressure at week 24. That was true of only 18.6% of placebo-treated controls, according to Dr. Hach of Boehringer Ingelheim Pharma in Ingelheim, Germany.
The impact of empagliflozin on cardiovascular events in patients with type 2 diabetes is under study in the ongoing EMPA-REG OUTCOME cardiovascular outcomes trial.
Empagliflozin is being jointly developed by Boehringer Ingelheim and Eli Lilly.
*Correction, 7/10/2013: An earlier version of the graphic accompanying this story incorrectly reported the change in uric acid.
CHICAGO – Twenty-four weeks of treatment with empagliflozin in patients with type 2 diabetes achieved clinically meaningful improvements in cardiovascular risk factors as well as in glycemic control, according to pooled data from four pivotal phase III clinical trials.
Empagliflozin is a sodium glucose cotransporter 2 (SGLT2) inhibitor. This is a novel emerging class of oral diabetic medications that work by boosting urinary glucose excretion. Canagliflozin (Invokana), the first-in-class SGLT2 inhibitor, received Food and Drug Administration marketing approval in March for treatment of type 2 diabetes. Empagliflozin’s application for marketing approval is now under review by the FDA and the European Medicines Agency, Dr. Thomas Hach noted at the annual scientific sessions of the American Diabetes Association.
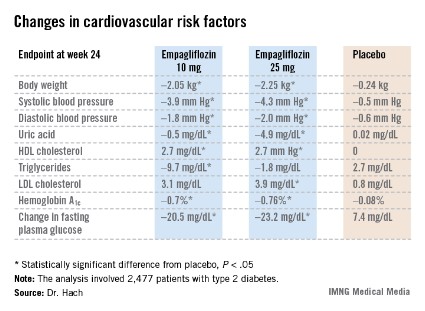
Empagliflozin resulted in significant, clinically meaningful improvements in blood pressure, body weight, uric acid, HDL cholesterol, hemoglobin A1c, and fasting plasma glucose, along with a modest, albeit unwelcome, increase in LDL cholesterol.
The improvement in blood pressure was particularly noteworthy. Among patients with uncontrolled blood pressure at baseline – that is, blood pressures of 130/80 mm Hg or higher – 33.3% of those who received empagliflozin at 10 mg/day and 32.2% on empagliflozin at 25 mg/day had controlled blood pressure at week 24. That was true of only 18.6% of placebo-treated controls, according to Dr. Hach of Boehringer Ingelheim Pharma in Ingelheim, Germany.
The impact of empagliflozin on cardiovascular events in patients with type 2 diabetes is under study in the ongoing EMPA-REG OUTCOME cardiovascular outcomes trial.
Empagliflozin is being jointly developed by Boehringer Ingelheim and Eli Lilly.
*Correction, 7/10/2013: An earlier version of the graphic accompanying this story incorrectly reported the change in uric acid.
CHICAGO – Twenty-four weeks of treatment with empagliflozin in patients with type 2 diabetes achieved clinically meaningful improvements in cardiovascular risk factors as well as in glycemic control, according to pooled data from four pivotal phase III clinical trials.
Empagliflozin is a sodium glucose cotransporter 2 (SGLT2) inhibitor. This is a novel emerging class of oral diabetic medications that work by boosting urinary glucose excretion. Canagliflozin (Invokana), the first-in-class SGLT2 inhibitor, received Food and Drug Administration marketing approval in March for treatment of type 2 diabetes. Empagliflozin’s application for marketing approval is now under review by the FDA and the European Medicines Agency, Dr. Thomas Hach noted at the annual scientific sessions of the American Diabetes Association.
Empagliflozin resulted in significant, clinically meaningful improvements in blood pressure, body weight, uric acid, HDL cholesterol, hemoglobin A1c, and fasting plasma glucose, along with a modest, albeit unwelcome, increase in LDL cholesterol.
The improvement in blood pressure was particularly noteworthy. Among patients with uncontrolled blood pressure at baseline – that is, blood pressures of 130/80 mm Hg or higher – 33.3% of those who received empagliflozin at 10 mg/day and 32.2% on empagliflozin at 25 mg/day had controlled blood pressure at week 24. That was true of only 18.6% of placebo-treated controls, according to Dr. Hach of Boehringer Ingelheim Pharma in Ingelheim, Germany.
The impact of empagliflozin on cardiovascular events in patients with type 2 diabetes is under study in the ongoing EMPA-REG OUTCOME cardiovascular outcomes trial.
Empagliflozin is being jointly developed by Boehringer Ingelheim and Eli Lilly.
*Correction, 7/10/2013: An earlier version of the graphic accompanying this story incorrectly reported the change in uric acid.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major Finding: One-third of type 2 diabetes patients with uncontrolled hypertension at baseline lowered their blood pressure to below 130/80 mm Hg after 24 weeks on empagliflozin.
Data Source: A pooled analysis of data from four randomized, placebo-controlled pivotal phase III clinical trials of empagliflozin in 2,477 patients with type 2 diabetes.
Disclosures: Empagliflozin is being jointly developed by Boehringer Ingelheim and Eli Lilly. Dr. Hach is an employee of Boehringer Ingelheim.