User login
In the U.S. about 2.7 to 6.1 million people have atrial fibrillation (AF).1 This condition affects the rhythm of the heart, causes blood in the heart to become stagnant, and puts patients at high risk for developing a systemic embolism, particularly a stroke.1 Recent studies have shown that AF accounts for at least 15% of all strokes in the U.S. and 36% of strokes in people aged > 80 years.2
For patients aged > 60 years, the gold standard of long-term anticoagulation for reducing the risk of stroke has been oral vitamin K antagonist (warfarin) therapy.2 Although overwhelming evidence exists that supports the use of warfarin in these patients, warfarin is a narrow therapeutic index medication that requires frequent laboratory monitoring of international normalized ratio (INR) for dose titration guidance. There is also strong evidence that pharmacist-run anticoagulation clinics have improved patient-centered outcomes in patients prescribed warfarin.3-5
Direct oral anticoagulants (DOACs) are recently approved oral medications used as alternatives to warfarin for anticoagulation in AF. Direct oral anticoagulants do not require INR monitoring or any laboratory test for efficacy. In 2010, the FDA approved the first DOAC, dabigatran, for use in patients with AF. In 2011, rivaroxaban received approval for the same indication. One potential drawback of these new agents relative to warfarin is the lack of availability of a reversal agent that can be used in the event of a life-threatening bleeding event. Dabigatran is the only DOAC with an FDA-approved available reversal agent. In both 2011 and 2012, dabigatran, warfarin, and other anticoagulants topped the Institute for Safe Medicine Practice list of suspect drugs related to adverse events (AEs). These data prompted the Joint Commission to incorporate anticoagulation into the 2017 National Hospital Patient Safety Goals to improve patient outcomes and reduce harm from use of anticoagulants.6
In early 2011, the VHA produced national guidance on the treatment of patients who receive DOACs; this guidance was updated most recently in September 2016.7 Patients who were receiving DOACs at the Ralph H. Johnson VAMC (RHJVAMC) were initially monitored by 12 primary care pharmacists at the main hospital or at community-based outpatient clinics (CBOCs). Ambulatory care pharmacists at RHJVAMC work under a scope of practice to prescribe and adjust certain classes of medications to provide the highest level of care to more than 65,000 veterans in South Carolina and Georgia. Historically at RHJVAMC, warfarin has been the anticoagulant most commonly used for AF, though dabigatran and rivaroxaban have gained in popularity after being added to the national VA formulary.
In November 2012, for better monitoring of patient outcomes, improved efficiency of the primary care pharmacist clinics, and increased access to care in these clinics, treatment of patients prescribed DOACs was shifted to a centralized model that involved 3 anticoagulation clinical pharmacy specialists.
Centralized pharmacy services have a small number of core team members in a specific service for a particular disease, which reduces the number of different pharmacists a patient could talk to for management of a particular condition. Centralized pharmacy services allow for streamlining anticoagulation management to a small group of individual pharmacists considered specialists in anticoagulation. This shift in management to centralized anticoagulation services was supported at RHJVAMC by findings from a study of a pharmacist-run centralized anticoagulation clinic: Patients treated by the centralized clinic were 39% less likely to experience an anticoagulation therapy complication.8
Protocol for dabigatran follow-up and monitoring at RHJVAMC was developed by clinical and supervisory pharmacy staff, to align with national VA guidance. When a provider determines a patient is a candidate for dabigatran, an outpatient consultation is entered for the clinical pharmacy specialist to review the appropriateness of the patient selection for therapy. If the patient is eligible for therapy, the pharmacist contacts the patient to set up an initial visit to confirm selection and to provide the first dabigatran prescription and counseling. For assessments, with specific emphasis on adherence and AE monitoring, the patient is contacted 2 weeks, 1 month, 3 months, and every 6 months after the initial appointment.
Although most of the literature supports pharmacist-managed anticoagulation for patients who receive warfarin, DOACs have become more integrated into practice and more evaluated. Evidence supports pharmacists' interventions on evaluation of patient education and dosing, but there is conflicting evidence regarding pharmacists' impact on adherence after 3 months of therapy.9,10 In a larger VA study of the impact of dabigatran adherence on patient-centered outcomes, patients were mostly nonadherent to prescribed dosing.11 These studies support the need for improved adherence in patients prescribed DOACs and the need for further investigation of pharmacists' roles in improving patient outcomes.
Methods
This single-center, retrospective anticoagulant-use evaluation covered 2 study periods between November 1, 2011 and October 31, 2013. Study approval was obtained from the institutional review board of the Medical University of South Carolina and the research and development committee of RHJVAMC. The study population consisted of veterans who had a diagnosis of AF and received at least 3 outpatient prescription fills of a 30-day supply of dabigatran at RHJVAMC during either or both of the study periods. Patients were excluded if they were pregnant or planning to become pregnant or were incarcerated at any time during the study period. Dabigatran was selected because it was the first DOAC added to the local VA formulary before the start of this study.
Patients who met the inclusion criteria were separated into 2 groups based on the dates of their prescription fills. The precentralization group included patients treated by primary care pharmacists from November 1, 2011 to October 31, 2012; the postcentralization group included patients treated by anticoagulation clinical pharmacy specialists from November 1, 2012 to October 31, 2013. In each group, patients were followed for 1 year during their respective study period. For analysis, patients were included in both study periods if they received at least 3 fills of dabigatran during each period.
Medication possession ratio (MPR), which was used to measure the primary endpoint of adherence, is defined as the proportion of days a patient had dabigatran. The MPR denominator is the total number of days between the first and last prescription refill dates within the 52-week study period; the numerator is calculated by summing the days' supply for all but the last filling of the medication during each respective period. Nonadherence was defined as an MPR < 0.8 (or 80%), which has been used to define poor adherence in the literature.12 The authors calculated all patients' mean MPRs and compared them to determine statistical significance by repeated-measures linear regression. Descriptive statistics on proportion of patients in each study group with MPR < 0.8 were examined. Last, the authors performed a comparative subanalysis of median MPRs to determine whether there was an adherence difference between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC.
The secondary focus of this study was safety outcomes, including any bleeding event or thromboembolism within either study period. A bleeding event was defined as any major or minor bleeding event recognized through ICD-9 codes or any bleeding recorded in the patient's chart and noted during chart review, as well as any serum hemoglobin (Hgb) level decrease of ≥ to 2 g/dL during the study period. Thromboembolism was defined as a thromboembolism recognized through ICD-9 codes or any thromboembolism noted during chart review. Descriptive statistics were reported for this outcome, and a chi-square test was used to compare bleeding events between groups to determine significance.
The tertiary focus of this study was clinical efficiency as determined by number of primary care pharmacist visits during each study period. Primary care pharmacist visits were included for all primary care pharmacists in primary care clinics at the main hospital and in all 6 CBOCs.
For statistical analysis α was set at 0.05, and P < .05 was considered statistically significant. SAS Enterprise Guide software (Cary, North Carolina) was used for all statistical analyses.
Results
An initial data pull was completed from the RHJVAMC prescription records database for patients who had ≥ 3 prescriptions of dabigatran filled for treatment of AF during the study period, which yielded 65 unique patients. There were 34 patients in the precentralization group and 55 patients in the postcentralization group. Twenty-four unique patients were included in both study groups.
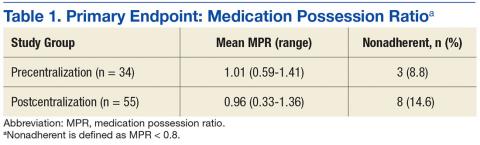
Mean MPR was 1.01 (range, 0.59-1.41) for the precentralization study period and 0.96 (range, 0.33-1.36) for the postcentralization period (Table 1). The difference was not statistically significant (P = .91). Number of patients considered nonadherent (MPR < 0.8) was 3 (8.82%) in the precentralization group and 8 (14.6%) in the postcentralization group.
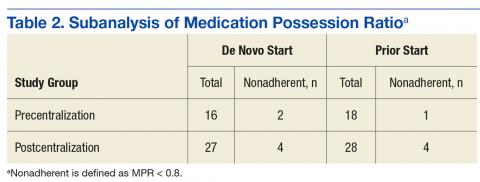
The primary endpoint subanalysis compared the median MPRs for the patients initially started on dabigatran at RHJVAMC (de novo starts) and the patients who were started on dabigatran before receiving it at RHJVAMC (prior starts). In each group, number and percentage of patients determined to be nonadherent by MPR were evaluated as well. De novo patients received initial assessment, counseling, and a dabigatran prescription from RHJVAMC pharmacists before or during the study period, and prior patients were initially prescribed dabigatran at another VA facility or at a non-VA facility (Table 2).
Regarding safety outcomes (secondary endpoint), a bleeding event was identified in 6 (17.7%) of the precentralization patients and 7 (12.7%) of the postcentralization patients. Of the 6 precentralization events, 1 was a case of hemoptysis, 1 was a hematoma on the forehead, 1 was a lower gastrointestinal bleed (unconfirmed), 1 was retinal hemorrhaging (noted by ophthalmologist), and 2 were serum Hgb level decreases of more than 2 g/dL (neither patient required transfusion of packed red blood cells). Of the 7 postcentralization events, 1 was persistent hematochezia caused by hemorrhoids, 1 was hematuria, 1 was a hematoma, 1 was an upper gastrointestinal bleed (required blood transfusion), and 4 were serum Hgb level decreases of more than 2 g/dL (1 of the 4 required transfusion). No precentralization patient had any evidence of thromboembolism during the study period; 1 postcentralization patient had a superficial venous thromboembolism near a hematoma on the elbow.
Discussion
In this single-center, retrospective medication-use evaluation, the authors found a high rate of adherence to dabigatran before and after centralization of outpatient DOAC management by pharmacists. There was no statistically significant difference in bleeding events between the study periods, but primary care pharmacist visits increased by 108% from precentralization to postcentralization. Although the primary outcome findings did not refute the study's null hypothesis, results support implementing centralized pharmacist DOAC management to maintain a high rate of adherence and a low incidence of adverse outcomes and providing more primary care pharmacist services to increase access to care for other chronic diseases.
Although there was no statistically significant difference in adherence rates between study periods, the 2 groups' rates were higher than the national average of 72%, as calculated by the proportion-of-days-covered (PDC) equation (median, 74%) in a 2015 large-scale study of site-level adherence in more than 5,000 VA patients.13 The authors' findings support that study's significant finding of a high rate of adherence to pharmacist-provided dabigatran treatment. This study's adherence rate also was higher than the median PDC rate reported in a 2014 study that focused on dabigatran adherence: 94% (mean, 84%; SD, 22%).11
The RHJVAMC follows national VA guidance on pharmacist follow-up for patients who receive DOACs. This follow-up focuses on frequent counseling over the first 6 months of de novo DOAC treatment and on monitoring and assessing adherence and AEs. Although there is less laboratory monitoring for DOAC treatment than for treatment with vitamin K antagonists (eg, warfarin), telephone monitoring as described in this study has been associated with a high adherence rate and minimization of AEs. The 2014 study with the 94% median PDC rate also showed an association of decreased adherence and increased harm, including combined all-cause mortality and stroke (hazard ratio, 1.13; 95% confidence interval [CI], 1.07-1.19 per 10% decrease in PDC rate).11
This study's subanalysis revealed no difference in adherence between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC. Each group had a high rate of adherence. Shore and colleagues found that most of the VA sites they surveyed (22/41) had anticoagulation clinics monitoring patients who were prescribed dabigatran.13 Pharmacist-led monitoring of adherence and AEs led to increased adherence to dabigatran treatment (relative risk, 1.25; 95% CI, 1.11-1.41), which was the standard of care at RHJVAMC throughout their entire study. Many of these factors may explain the very high rate of adherence found in the present study, specifically in comparison to previously reported national averages.
In addition, the authors found no statistically significant difference in bleeding outcomes between the precentralization and postcentralization groups. Their incidence of bleeding was similar to the 16.6% rate reported in the package insert for dabigatran.14 Furthermore, the safety outcomes were similar for both groups in this study, which may be attributable to the quality of patient care provided by all RHJVAMC pharmacists, particularly in the setting of dabigatran management.
Many studies have found an association between dabigatran use and an increased rate of bleeding, particularly gastrointestinal, as demonstrated in several patients in this study. Evidence of these clinically significant AEs further supports pharmacists' close monitoring to detect these AEs and working with patients' providers to determine whether an alternative anticoagulant should be used.
A significant finding of this study regarding centralization of DOAC management by pharmacists was the increased number of primary care pharmacist visits. By streamlining all anticoagulant services to anticoagulation clinical pharmacy specialists, primary care pharmacists were able to care for more veterans and increase access to care without adding staff. The centralized anticoagulation pharmacists were volunteers who held other positions within the department; they did not have to be replaced when they became anticoagulation providers. This workload reallocation helped the RHJVAMC pharmacy department increase access to care.
Limitations
This study had several potential limitations. First, MPR, a widely studied common tool for assessing adherence, has been criticized for often being imprecise when used with short study periods.12 Another commonly used adherence measure is PDC rate, which has been reported in several large-scale studies of dabigatran therapy. The authors selected MPR for the present study because MPR calculation is more practical in the patient population and because MPR and PDC rate are predicted to yield similar results in assessments of adherence to a single medication.12 It also should be noted that both MPR and PDC rate are surrogate markers for adherence and assume adherence based on the availability of medication to the patient. Assessing adherence in a retrospective study is a challenge, as more reliable adherence assessment--for example, with use of pill counts or blister packs--is not possible. This study's retrospective design was another potential limitation, as an active intervention was not used.
In addition, this study had a small sample, likely attributable to the addition of dabigatran to the VA national formulary just months before the start of the study period. Furthermore, this study was not powered to detect significant differences in safety or efficacy outcomes. Other potential study limitations included having national VA guidance regarding follow-up periods and dabigatran prescription quantity limits during both study periods. Also, there was some potential for pharmacist-initiated refills at follow-up visits, which could falsely increase MPR. Last, the study analyzed only 1 DOAC and not the entire class of medications.
Conclusion
Centralizing DOAC management by clinical pharmacy specialists at a single VA facility helped maintain high rates of dabigatran adherence, above the national average, and low rates of adverse outcomes were maintained in both study groups. In addition, centralization of anticoagulation services improved access to care through an increase in primary care pharmacist visits without the addition of staff. Centralization of DOAC management by pharmacists is a viable option for maintaining high rates of adherence and low rates of adverse outcomes in facilities where the goal is to achieve clinical efficiency.
1. January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society [published correction appears in J Am Coll Cardiol. 2014;64(21):2305-2307. J Am Coll Cardiol. 2014;64(21):e1-e76.
2. Reiffel JA. New versus traditional approaches to oral anticoagulation in patients with atrial fibrillation. Am J Med. 2014;127(4):e15.
3. Locke C, Ravnan SL, Patel R, Uchizono JA. Reduction in warfarin adverse events requiring patient hospitalization after implementation of pharmacist-managed anticoagulation service. Pharmacotherapy. 2005;25(5):685-689.
4. Poon IO, Lal L, Brown EN, Braun UK. The impact of pharmacist-managed oral anticoagulation therapy in older veterans. J Clin Pharm Ther. 2007;32(1):21-29.
5. Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care. Arch Intern Med. 1998;158(15):1641-1647.
6. The Joint Commission. National patient safety goals. https://www.jointcommission.org/as sets/1/6/2017_NPSG_HAP_ER.pdf. Published 2016. Accessed December 6, 2016.
7. Department of Veterans Affairs Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis): Criteria for Use for Stroke Prevention in nonvalvular atrial fibrillation (AF) and Edoxaban (SAVAYSA). http://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse/Anticoagulants_Direct_Oral_DOACs_CFU_and_Algorithm_for_Nonvalvular_Atrial_Fibrillation_Sep_2016.pdf. Updated September 2016. Accessed December 6, 2016.
8. Witt DM, Sadler MA, Shanahan RL, Mazzoli G, Tillman DJ. Effect of a centralized clinical pharmacy anticoagulation service on the outcomes of anticoagulation therapy. Chest. 2005;127(5):1515-1522.
9. Chan LL, Crumpler WL, Jacobson AK. Implementation of pharmacist-managed anticoagulation in patients receiving newer anticoagulants. Am J Health Syst Pharm. 2013;70(15):1285-1286, 1288.
10. Lee PY, Han SY, Miyahara RK. Adherence and outcomes of patients treated with dabigatran: pharmacist-managed anticoagulation clinic versus usual care. Am J Health Syst Pharm. 2013;70(13):1154-1161.
11. Shore S, Carey EP, Turakhia MP, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the Veterans Health Administration. Am Heart J. 2014;167(6):810-817.
12. Martin BC, Wiley-Exley EK, Richards S, Domino ME, Carey TS, Sleath BL. Contrasting measures of adherence with simple drug use, medication switching and therapeutic duplication. Ann Pharmacother. 2009;43(1):36-44.
13. Shore S, Ho PM, Lambert-Kerzner A, et al. Site-level variation in and practices associated with dabigatran adherence. JAMA. 2015;313(14):1443-1450.
14. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2015.
In the U.S. about 2.7 to 6.1 million people have atrial fibrillation (AF).1 This condition affects the rhythm of the heart, causes blood in the heart to become stagnant, and puts patients at high risk for developing a systemic embolism, particularly a stroke.1 Recent studies have shown that AF accounts for at least 15% of all strokes in the U.S. and 36% of strokes in people aged > 80 years.2
For patients aged > 60 years, the gold standard of long-term anticoagulation for reducing the risk of stroke has been oral vitamin K antagonist (warfarin) therapy.2 Although overwhelming evidence exists that supports the use of warfarin in these patients, warfarin is a narrow therapeutic index medication that requires frequent laboratory monitoring of international normalized ratio (INR) for dose titration guidance. There is also strong evidence that pharmacist-run anticoagulation clinics have improved patient-centered outcomes in patients prescribed warfarin.3-5
Direct oral anticoagulants (DOACs) are recently approved oral medications used as alternatives to warfarin for anticoagulation in AF. Direct oral anticoagulants do not require INR monitoring or any laboratory test for efficacy. In 2010, the FDA approved the first DOAC, dabigatran, for use in patients with AF. In 2011, rivaroxaban received approval for the same indication. One potential drawback of these new agents relative to warfarin is the lack of availability of a reversal agent that can be used in the event of a life-threatening bleeding event. Dabigatran is the only DOAC with an FDA-approved available reversal agent. In both 2011 and 2012, dabigatran, warfarin, and other anticoagulants topped the Institute for Safe Medicine Practice list of suspect drugs related to adverse events (AEs). These data prompted the Joint Commission to incorporate anticoagulation into the 2017 National Hospital Patient Safety Goals to improve patient outcomes and reduce harm from use of anticoagulants.6
In early 2011, the VHA produced national guidance on the treatment of patients who receive DOACs; this guidance was updated most recently in September 2016.7 Patients who were receiving DOACs at the Ralph H. Johnson VAMC (RHJVAMC) were initially monitored by 12 primary care pharmacists at the main hospital or at community-based outpatient clinics (CBOCs). Ambulatory care pharmacists at RHJVAMC work under a scope of practice to prescribe and adjust certain classes of medications to provide the highest level of care to more than 65,000 veterans in South Carolina and Georgia. Historically at RHJVAMC, warfarin has been the anticoagulant most commonly used for AF, though dabigatran and rivaroxaban have gained in popularity after being added to the national VA formulary.
In November 2012, for better monitoring of patient outcomes, improved efficiency of the primary care pharmacist clinics, and increased access to care in these clinics, treatment of patients prescribed DOACs was shifted to a centralized model that involved 3 anticoagulation clinical pharmacy specialists.
Centralized pharmacy services have a small number of core team members in a specific service for a particular disease, which reduces the number of different pharmacists a patient could talk to for management of a particular condition. Centralized pharmacy services allow for streamlining anticoagulation management to a small group of individual pharmacists considered specialists in anticoagulation. This shift in management to centralized anticoagulation services was supported at RHJVAMC by findings from a study of a pharmacist-run centralized anticoagulation clinic: Patients treated by the centralized clinic were 39% less likely to experience an anticoagulation therapy complication.8
Protocol for dabigatran follow-up and monitoring at RHJVAMC was developed by clinical and supervisory pharmacy staff, to align with national VA guidance. When a provider determines a patient is a candidate for dabigatran, an outpatient consultation is entered for the clinical pharmacy specialist to review the appropriateness of the patient selection for therapy. If the patient is eligible for therapy, the pharmacist contacts the patient to set up an initial visit to confirm selection and to provide the first dabigatran prescription and counseling. For assessments, with specific emphasis on adherence and AE monitoring, the patient is contacted 2 weeks, 1 month, 3 months, and every 6 months after the initial appointment.
Although most of the literature supports pharmacist-managed anticoagulation for patients who receive warfarin, DOACs have become more integrated into practice and more evaluated. Evidence supports pharmacists' interventions on evaluation of patient education and dosing, but there is conflicting evidence regarding pharmacists' impact on adherence after 3 months of therapy.9,10 In a larger VA study of the impact of dabigatran adherence on patient-centered outcomes, patients were mostly nonadherent to prescribed dosing.11 These studies support the need for improved adherence in patients prescribed DOACs and the need for further investigation of pharmacists' roles in improving patient outcomes.
Methods
This single-center, retrospective anticoagulant-use evaluation covered 2 study periods between November 1, 2011 and October 31, 2013. Study approval was obtained from the institutional review board of the Medical University of South Carolina and the research and development committee of RHJVAMC. The study population consisted of veterans who had a diagnosis of AF and received at least 3 outpatient prescription fills of a 30-day supply of dabigatran at RHJVAMC during either or both of the study periods. Patients were excluded if they were pregnant or planning to become pregnant or were incarcerated at any time during the study period. Dabigatran was selected because it was the first DOAC added to the local VA formulary before the start of this study.
Patients who met the inclusion criteria were separated into 2 groups based on the dates of their prescription fills. The precentralization group included patients treated by primary care pharmacists from November 1, 2011 to October 31, 2012; the postcentralization group included patients treated by anticoagulation clinical pharmacy specialists from November 1, 2012 to October 31, 2013. In each group, patients were followed for 1 year during their respective study period. For analysis, patients were included in both study periods if they received at least 3 fills of dabigatran during each period.
Medication possession ratio (MPR), which was used to measure the primary endpoint of adherence, is defined as the proportion of days a patient had dabigatran. The MPR denominator is the total number of days between the first and last prescription refill dates within the 52-week study period; the numerator is calculated by summing the days' supply for all but the last filling of the medication during each respective period. Nonadherence was defined as an MPR < 0.8 (or 80%), which has been used to define poor adherence in the literature.12 The authors calculated all patients' mean MPRs and compared them to determine statistical significance by repeated-measures linear regression. Descriptive statistics on proportion of patients in each study group with MPR < 0.8 were examined. Last, the authors performed a comparative subanalysis of median MPRs to determine whether there was an adherence difference between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC.
The secondary focus of this study was safety outcomes, including any bleeding event or thromboembolism within either study period. A bleeding event was defined as any major or minor bleeding event recognized through ICD-9 codes or any bleeding recorded in the patient's chart and noted during chart review, as well as any serum hemoglobin (Hgb) level decrease of ≥ to 2 g/dL during the study period. Thromboembolism was defined as a thromboembolism recognized through ICD-9 codes or any thromboembolism noted during chart review. Descriptive statistics were reported for this outcome, and a chi-square test was used to compare bleeding events between groups to determine significance.
The tertiary focus of this study was clinical efficiency as determined by number of primary care pharmacist visits during each study period. Primary care pharmacist visits were included for all primary care pharmacists in primary care clinics at the main hospital and in all 6 CBOCs.
For statistical analysis α was set at 0.05, and P < .05 was considered statistically significant. SAS Enterprise Guide software (Cary, North Carolina) was used for all statistical analyses.
Results
An initial data pull was completed from the RHJVAMC prescription records database for patients who had ≥ 3 prescriptions of dabigatran filled for treatment of AF during the study period, which yielded 65 unique patients. There were 34 patients in the precentralization group and 55 patients in the postcentralization group. Twenty-four unique patients were included in both study groups.
Mean MPR was 1.01 (range, 0.59-1.41) for the precentralization study period and 0.96 (range, 0.33-1.36) for the postcentralization period (Table 1). The difference was not statistically significant (P = .91). Number of patients considered nonadherent (MPR < 0.8) was 3 (8.82%) in the precentralization group and 8 (14.6%) in the postcentralization group.
The primary endpoint subanalysis compared the median MPRs for the patients initially started on dabigatran at RHJVAMC (de novo starts) and the patients who were started on dabigatran before receiving it at RHJVAMC (prior starts). In each group, number and percentage of patients determined to be nonadherent by MPR were evaluated as well. De novo patients received initial assessment, counseling, and a dabigatran prescription from RHJVAMC pharmacists before or during the study period, and prior patients were initially prescribed dabigatran at another VA facility or at a non-VA facility (Table 2).
Regarding safety outcomes (secondary endpoint), a bleeding event was identified in 6 (17.7%) of the precentralization patients and 7 (12.7%) of the postcentralization patients. Of the 6 precentralization events, 1 was a case of hemoptysis, 1 was a hematoma on the forehead, 1 was a lower gastrointestinal bleed (unconfirmed), 1 was retinal hemorrhaging (noted by ophthalmologist), and 2 were serum Hgb level decreases of more than 2 g/dL (neither patient required transfusion of packed red blood cells). Of the 7 postcentralization events, 1 was persistent hematochezia caused by hemorrhoids, 1 was hematuria, 1 was a hematoma, 1 was an upper gastrointestinal bleed (required blood transfusion), and 4 were serum Hgb level decreases of more than 2 g/dL (1 of the 4 required transfusion). No precentralization patient had any evidence of thromboembolism during the study period; 1 postcentralization patient had a superficial venous thromboembolism near a hematoma on the elbow.
Discussion
In this single-center, retrospective medication-use evaluation, the authors found a high rate of adherence to dabigatran before and after centralization of outpatient DOAC management by pharmacists. There was no statistically significant difference in bleeding events between the study periods, but primary care pharmacist visits increased by 108% from precentralization to postcentralization. Although the primary outcome findings did not refute the study's null hypothesis, results support implementing centralized pharmacist DOAC management to maintain a high rate of adherence and a low incidence of adverse outcomes and providing more primary care pharmacist services to increase access to care for other chronic diseases.
Although there was no statistically significant difference in adherence rates between study periods, the 2 groups' rates were higher than the national average of 72%, as calculated by the proportion-of-days-covered (PDC) equation (median, 74%) in a 2015 large-scale study of site-level adherence in more than 5,000 VA patients.13 The authors' findings support that study's significant finding of a high rate of adherence to pharmacist-provided dabigatran treatment. This study's adherence rate also was higher than the median PDC rate reported in a 2014 study that focused on dabigatran adherence: 94% (mean, 84%; SD, 22%).11
The RHJVAMC follows national VA guidance on pharmacist follow-up for patients who receive DOACs. This follow-up focuses on frequent counseling over the first 6 months of de novo DOAC treatment and on monitoring and assessing adherence and AEs. Although there is less laboratory monitoring for DOAC treatment than for treatment with vitamin K antagonists (eg, warfarin), telephone monitoring as described in this study has been associated with a high adherence rate and minimization of AEs. The 2014 study with the 94% median PDC rate also showed an association of decreased adherence and increased harm, including combined all-cause mortality and stroke (hazard ratio, 1.13; 95% confidence interval [CI], 1.07-1.19 per 10% decrease in PDC rate).11
This study's subanalysis revealed no difference in adherence between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC. Each group had a high rate of adherence. Shore and colleagues found that most of the VA sites they surveyed (22/41) had anticoagulation clinics monitoring patients who were prescribed dabigatran.13 Pharmacist-led monitoring of adherence and AEs led to increased adherence to dabigatran treatment (relative risk, 1.25; 95% CI, 1.11-1.41), which was the standard of care at RHJVAMC throughout their entire study. Many of these factors may explain the very high rate of adherence found in the present study, specifically in comparison to previously reported national averages.
In addition, the authors found no statistically significant difference in bleeding outcomes between the precentralization and postcentralization groups. Their incidence of bleeding was similar to the 16.6% rate reported in the package insert for dabigatran.14 Furthermore, the safety outcomes were similar for both groups in this study, which may be attributable to the quality of patient care provided by all RHJVAMC pharmacists, particularly in the setting of dabigatran management.
Many studies have found an association between dabigatran use and an increased rate of bleeding, particularly gastrointestinal, as demonstrated in several patients in this study. Evidence of these clinically significant AEs further supports pharmacists' close monitoring to detect these AEs and working with patients' providers to determine whether an alternative anticoagulant should be used.
A significant finding of this study regarding centralization of DOAC management by pharmacists was the increased number of primary care pharmacist visits. By streamlining all anticoagulant services to anticoagulation clinical pharmacy specialists, primary care pharmacists were able to care for more veterans and increase access to care without adding staff. The centralized anticoagulation pharmacists were volunteers who held other positions within the department; they did not have to be replaced when they became anticoagulation providers. This workload reallocation helped the RHJVAMC pharmacy department increase access to care.
Limitations
This study had several potential limitations. First, MPR, a widely studied common tool for assessing adherence, has been criticized for often being imprecise when used with short study periods.12 Another commonly used adherence measure is PDC rate, which has been reported in several large-scale studies of dabigatran therapy. The authors selected MPR for the present study because MPR calculation is more practical in the patient population and because MPR and PDC rate are predicted to yield similar results in assessments of adherence to a single medication.12 It also should be noted that both MPR and PDC rate are surrogate markers for adherence and assume adherence based on the availability of medication to the patient. Assessing adherence in a retrospective study is a challenge, as more reliable adherence assessment--for example, with use of pill counts or blister packs--is not possible. This study's retrospective design was another potential limitation, as an active intervention was not used.
In addition, this study had a small sample, likely attributable to the addition of dabigatran to the VA national formulary just months before the start of the study period. Furthermore, this study was not powered to detect significant differences in safety or efficacy outcomes. Other potential study limitations included having national VA guidance regarding follow-up periods and dabigatran prescription quantity limits during both study periods. Also, there was some potential for pharmacist-initiated refills at follow-up visits, which could falsely increase MPR. Last, the study analyzed only 1 DOAC and not the entire class of medications.
Conclusion
Centralizing DOAC management by clinical pharmacy specialists at a single VA facility helped maintain high rates of dabigatran adherence, above the national average, and low rates of adverse outcomes were maintained in both study groups. In addition, centralization of anticoagulation services improved access to care through an increase in primary care pharmacist visits without the addition of staff. Centralization of DOAC management by pharmacists is a viable option for maintaining high rates of adherence and low rates of adverse outcomes in facilities where the goal is to achieve clinical efficiency.
In the U.S. about 2.7 to 6.1 million people have atrial fibrillation (AF).1 This condition affects the rhythm of the heart, causes blood in the heart to become stagnant, and puts patients at high risk for developing a systemic embolism, particularly a stroke.1 Recent studies have shown that AF accounts for at least 15% of all strokes in the U.S. and 36% of strokes in people aged > 80 years.2
For patients aged > 60 years, the gold standard of long-term anticoagulation for reducing the risk of stroke has been oral vitamin K antagonist (warfarin) therapy.2 Although overwhelming evidence exists that supports the use of warfarin in these patients, warfarin is a narrow therapeutic index medication that requires frequent laboratory monitoring of international normalized ratio (INR) for dose titration guidance. There is also strong evidence that pharmacist-run anticoagulation clinics have improved patient-centered outcomes in patients prescribed warfarin.3-5
Direct oral anticoagulants (DOACs) are recently approved oral medications used as alternatives to warfarin for anticoagulation in AF. Direct oral anticoagulants do not require INR monitoring or any laboratory test for efficacy. In 2010, the FDA approved the first DOAC, dabigatran, for use in patients with AF. In 2011, rivaroxaban received approval for the same indication. One potential drawback of these new agents relative to warfarin is the lack of availability of a reversal agent that can be used in the event of a life-threatening bleeding event. Dabigatran is the only DOAC with an FDA-approved available reversal agent. In both 2011 and 2012, dabigatran, warfarin, and other anticoagulants topped the Institute for Safe Medicine Practice list of suspect drugs related to adverse events (AEs). These data prompted the Joint Commission to incorporate anticoagulation into the 2017 National Hospital Patient Safety Goals to improve patient outcomes and reduce harm from use of anticoagulants.6
In early 2011, the VHA produced national guidance on the treatment of patients who receive DOACs; this guidance was updated most recently in September 2016.7 Patients who were receiving DOACs at the Ralph H. Johnson VAMC (RHJVAMC) were initially monitored by 12 primary care pharmacists at the main hospital or at community-based outpatient clinics (CBOCs). Ambulatory care pharmacists at RHJVAMC work under a scope of practice to prescribe and adjust certain classes of medications to provide the highest level of care to more than 65,000 veterans in South Carolina and Georgia. Historically at RHJVAMC, warfarin has been the anticoagulant most commonly used for AF, though dabigatran and rivaroxaban have gained in popularity after being added to the national VA formulary.
In November 2012, for better monitoring of patient outcomes, improved efficiency of the primary care pharmacist clinics, and increased access to care in these clinics, treatment of patients prescribed DOACs was shifted to a centralized model that involved 3 anticoagulation clinical pharmacy specialists.
Centralized pharmacy services have a small number of core team members in a specific service for a particular disease, which reduces the number of different pharmacists a patient could talk to for management of a particular condition. Centralized pharmacy services allow for streamlining anticoagulation management to a small group of individual pharmacists considered specialists in anticoagulation. This shift in management to centralized anticoagulation services was supported at RHJVAMC by findings from a study of a pharmacist-run centralized anticoagulation clinic: Patients treated by the centralized clinic were 39% less likely to experience an anticoagulation therapy complication.8
Protocol for dabigatran follow-up and monitoring at RHJVAMC was developed by clinical and supervisory pharmacy staff, to align with national VA guidance. When a provider determines a patient is a candidate for dabigatran, an outpatient consultation is entered for the clinical pharmacy specialist to review the appropriateness of the patient selection for therapy. If the patient is eligible for therapy, the pharmacist contacts the patient to set up an initial visit to confirm selection and to provide the first dabigatran prescription and counseling. For assessments, with specific emphasis on adherence and AE monitoring, the patient is contacted 2 weeks, 1 month, 3 months, and every 6 months after the initial appointment.
Although most of the literature supports pharmacist-managed anticoagulation for patients who receive warfarin, DOACs have become more integrated into practice and more evaluated. Evidence supports pharmacists' interventions on evaluation of patient education and dosing, but there is conflicting evidence regarding pharmacists' impact on adherence after 3 months of therapy.9,10 In a larger VA study of the impact of dabigatran adherence on patient-centered outcomes, patients were mostly nonadherent to prescribed dosing.11 These studies support the need for improved adherence in patients prescribed DOACs and the need for further investigation of pharmacists' roles in improving patient outcomes.
Methods
This single-center, retrospective anticoagulant-use evaluation covered 2 study periods between November 1, 2011 and October 31, 2013. Study approval was obtained from the institutional review board of the Medical University of South Carolina and the research and development committee of RHJVAMC. The study population consisted of veterans who had a diagnosis of AF and received at least 3 outpatient prescription fills of a 30-day supply of dabigatran at RHJVAMC during either or both of the study periods. Patients were excluded if they were pregnant or planning to become pregnant or were incarcerated at any time during the study period. Dabigatran was selected because it was the first DOAC added to the local VA formulary before the start of this study.
Patients who met the inclusion criteria were separated into 2 groups based on the dates of their prescription fills. The precentralization group included patients treated by primary care pharmacists from November 1, 2011 to October 31, 2012; the postcentralization group included patients treated by anticoagulation clinical pharmacy specialists from November 1, 2012 to October 31, 2013. In each group, patients were followed for 1 year during their respective study period. For analysis, patients were included in both study periods if they received at least 3 fills of dabigatran during each period.
Medication possession ratio (MPR), which was used to measure the primary endpoint of adherence, is defined as the proportion of days a patient had dabigatran. The MPR denominator is the total number of days between the first and last prescription refill dates within the 52-week study period; the numerator is calculated by summing the days' supply for all but the last filling of the medication during each respective period. Nonadherence was defined as an MPR < 0.8 (or 80%), which has been used to define poor adherence in the literature.12 The authors calculated all patients' mean MPRs and compared them to determine statistical significance by repeated-measures linear regression. Descriptive statistics on proportion of patients in each study group with MPR < 0.8 were examined. Last, the authors performed a comparative subanalysis of median MPRs to determine whether there was an adherence difference between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC.
The secondary focus of this study was safety outcomes, including any bleeding event or thromboembolism within either study period. A bleeding event was defined as any major or minor bleeding event recognized through ICD-9 codes or any bleeding recorded in the patient's chart and noted during chart review, as well as any serum hemoglobin (Hgb) level decrease of ≥ to 2 g/dL during the study period. Thromboembolism was defined as a thromboembolism recognized through ICD-9 codes or any thromboembolism noted during chart review. Descriptive statistics were reported for this outcome, and a chi-square test was used to compare bleeding events between groups to determine significance.
The tertiary focus of this study was clinical efficiency as determined by number of primary care pharmacist visits during each study period. Primary care pharmacist visits were included for all primary care pharmacists in primary care clinics at the main hospital and in all 6 CBOCs.
For statistical analysis α was set at 0.05, and P < .05 was considered statistically significant. SAS Enterprise Guide software (Cary, North Carolina) was used for all statistical analyses.
Results
An initial data pull was completed from the RHJVAMC prescription records database for patients who had ≥ 3 prescriptions of dabigatran filled for treatment of AF during the study period, which yielded 65 unique patients. There were 34 patients in the precentralization group and 55 patients in the postcentralization group. Twenty-four unique patients were included in both study groups.
Mean MPR was 1.01 (range, 0.59-1.41) for the precentralization study period and 0.96 (range, 0.33-1.36) for the postcentralization period (Table 1). The difference was not statistically significant (P = .91). Number of patients considered nonadherent (MPR < 0.8) was 3 (8.82%) in the precentralization group and 8 (14.6%) in the postcentralization group.
The primary endpoint subanalysis compared the median MPRs for the patients initially started on dabigatran at RHJVAMC (de novo starts) and the patients who were started on dabigatran before receiving it at RHJVAMC (prior starts). In each group, number and percentage of patients determined to be nonadherent by MPR were evaluated as well. De novo patients received initial assessment, counseling, and a dabigatran prescription from RHJVAMC pharmacists before or during the study period, and prior patients were initially prescribed dabigatran at another VA facility or at a non-VA facility (Table 2).
Regarding safety outcomes (secondary endpoint), a bleeding event was identified in 6 (17.7%) of the precentralization patients and 7 (12.7%) of the postcentralization patients. Of the 6 precentralization events, 1 was a case of hemoptysis, 1 was a hematoma on the forehead, 1 was a lower gastrointestinal bleed (unconfirmed), 1 was retinal hemorrhaging (noted by ophthalmologist), and 2 were serum Hgb level decreases of more than 2 g/dL (neither patient required transfusion of packed red blood cells). Of the 7 postcentralization events, 1 was persistent hematochezia caused by hemorrhoids, 1 was hematuria, 1 was a hematoma, 1 was an upper gastrointestinal bleed (required blood transfusion), and 4 were serum Hgb level decreases of more than 2 g/dL (1 of the 4 required transfusion). No precentralization patient had any evidence of thromboembolism during the study period; 1 postcentralization patient had a superficial venous thromboembolism near a hematoma on the elbow.
Discussion
In this single-center, retrospective medication-use evaluation, the authors found a high rate of adherence to dabigatran before and after centralization of outpatient DOAC management by pharmacists. There was no statistically significant difference in bleeding events between the study periods, but primary care pharmacist visits increased by 108% from precentralization to postcentralization. Although the primary outcome findings did not refute the study's null hypothesis, results support implementing centralized pharmacist DOAC management to maintain a high rate of adherence and a low incidence of adverse outcomes and providing more primary care pharmacist services to increase access to care for other chronic diseases.
Although there was no statistically significant difference in adherence rates between study periods, the 2 groups' rates were higher than the national average of 72%, as calculated by the proportion-of-days-covered (PDC) equation (median, 74%) in a 2015 large-scale study of site-level adherence in more than 5,000 VA patients.13 The authors' findings support that study's significant finding of a high rate of adherence to pharmacist-provided dabigatran treatment. This study's adherence rate also was higher than the median PDC rate reported in a 2014 study that focused on dabigatran adherence: 94% (mean, 84%; SD, 22%).11
The RHJVAMC follows national VA guidance on pharmacist follow-up for patients who receive DOACs. This follow-up focuses on frequent counseling over the first 6 months of de novo DOAC treatment and on monitoring and assessing adherence and AEs. Although there is less laboratory monitoring for DOAC treatment than for treatment with vitamin K antagonists (eg, warfarin), telephone monitoring as described in this study has been associated with a high adherence rate and minimization of AEs. The 2014 study with the 94% median PDC rate also showed an association of decreased adherence and increased harm, including combined all-cause mortality and stroke (hazard ratio, 1.13; 95% confidence interval [CI], 1.07-1.19 per 10% decrease in PDC rate).11
This study's subanalysis revealed no difference in adherence between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC. Each group had a high rate of adherence. Shore and colleagues found that most of the VA sites they surveyed (22/41) had anticoagulation clinics monitoring patients who were prescribed dabigatran.13 Pharmacist-led monitoring of adherence and AEs led to increased adherence to dabigatran treatment (relative risk, 1.25; 95% CI, 1.11-1.41), which was the standard of care at RHJVAMC throughout their entire study. Many of these factors may explain the very high rate of adherence found in the present study, specifically in comparison to previously reported national averages.
In addition, the authors found no statistically significant difference in bleeding outcomes between the precentralization and postcentralization groups. Their incidence of bleeding was similar to the 16.6% rate reported in the package insert for dabigatran.14 Furthermore, the safety outcomes were similar for both groups in this study, which may be attributable to the quality of patient care provided by all RHJVAMC pharmacists, particularly in the setting of dabigatran management.
Many studies have found an association between dabigatran use and an increased rate of bleeding, particularly gastrointestinal, as demonstrated in several patients in this study. Evidence of these clinically significant AEs further supports pharmacists' close monitoring to detect these AEs and working with patients' providers to determine whether an alternative anticoagulant should be used.
A significant finding of this study regarding centralization of DOAC management by pharmacists was the increased number of primary care pharmacist visits. By streamlining all anticoagulant services to anticoagulation clinical pharmacy specialists, primary care pharmacists were able to care for more veterans and increase access to care without adding staff. The centralized anticoagulation pharmacists were volunteers who held other positions within the department; they did not have to be replaced when they became anticoagulation providers. This workload reallocation helped the RHJVAMC pharmacy department increase access to care.
Limitations
This study had several potential limitations. First, MPR, a widely studied common tool for assessing adherence, has been criticized for often being imprecise when used with short study periods.12 Another commonly used adherence measure is PDC rate, which has been reported in several large-scale studies of dabigatran therapy. The authors selected MPR for the present study because MPR calculation is more practical in the patient population and because MPR and PDC rate are predicted to yield similar results in assessments of adherence to a single medication.12 It also should be noted that both MPR and PDC rate are surrogate markers for adherence and assume adherence based on the availability of medication to the patient. Assessing adherence in a retrospective study is a challenge, as more reliable adherence assessment--for example, with use of pill counts or blister packs--is not possible. This study's retrospective design was another potential limitation, as an active intervention was not used.
In addition, this study had a small sample, likely attributable to the addition of dabigatran to the VA national formulary just months before the start of the study period. Furthermore, this study was not powered to detect significant differences in safety or efficacy outcomes. Other potential study limitations included having national VA guidance regarding follow-up periods and dabigatran prescription quantity limits during both study periods. Also, there was some potential for pharmacist-initiated refills at follow-up visits, which could falsely increase MPR. Last, the study analyzed only 1 DOAC and not the entire class of medications.
Conclusion
Centralizing DOAC management by clinical pharmacy specialists at a single VA facility helped maintain high rates of dabigatran adherence, above the national average, and low rates of adverse outcomes were maintained in both study groups. In addition, centralization of anticoagulation services improved access to care through an increase in primary care pharmacist visits without the addition of staff. Centralization of DOAC management by pharmacists is a viable option for maintaining high rates of adherence and low rates of adverse outcomes in facilities where the goal is to achieve clinical efficiency.
1. January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society [published correction appears in J Am Coll Cardiol. 2014;64(21):2305-2307. J Am Coll Cardiol. 2014;64(21):e1-e76.
2. Reiffel JA. New versus traditional approaches to oral anticoagulation in patients with atrial fibrillation. Am J Med. 2014;127(4):e15.
3. Locke C, Ravnan SL, Patel R, Uchizono JA. Reduction in warfarin adverse events requiring patient hospitalization after implementation of pharmacist-managed anticoagulation service. Pharmacotherapy. 2005;25(5):685-689.
4. Poon IO, Lal L, Brown EN, Braun UK. The impact of pharmacist-managed oral anticoagulation therapy in older veterans. J Clin Pharm Ther. 2007;32(1):21-29.
5. Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care. Arch Intern Med. 1998;158(15):1641-1647.
6. The Joint Commission. National patient safety goals. https://www.jointcommission.org/as sets/1/6/2017_NPSG_HAP_ER.pdf. Published 2016. Accessed December 6, 2016.
7. Department of Veterans Affairs Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis): Criteria for Use for Stroke Prevention in nonvalvular atrial fibrillation (AF) and Edoxaban (SAVAYSA). http://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse/Anticoagulants_Direct_Oral_DOACs_CFU_and_Algorithm_for_Nonvalvular_Atrial_Fibrillation_Sep_2016.pdf. Updated September 2016. Accessed December 6, 2016.
8. Witt DM, Sadler MA, Shanahan RL, Mazzoli G, Tillman DJ. Effect of a centralized clinical pharmacy anticoagulation service on the outcomes of anticoagulation therapy. Chest. 2005;127(5):1515-1522.
9. Chan LL, Crumpler WL, Jacobson AK. Implementation of pharmacist-managed anticoagulation in patients receiving newer anticoagulants. Am J Health Syst Pharm. 2013;70(15):1285-1286, 1288.
10. Lee PY, Han SY, Miyahara RK. Adherence and outcomes of patients treated with dabigatran: pharmacist-managed anticoagulation clinic versus usual care. Am J Health Syst Pharm. 2013;70(13):1154-1161.
11. Shore S, Carey EP, Turakhia MP, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the Veterans Health Administration. Am Heart J. 2014;167(6):810-817.
12. Martin BC, Wiley-Exley EK, Richards S, Domino ME, Carey TS, Sleath BL. Contrasting measures of adherence with simple drug use, medication switching and therapeutic duplication. Ann Pharmacother. 2009;43(1):36-44.
13. Shore S, Ho PM, Lambert-Kerzner A, et al. Site-level variation in and practices associated with dabigatran adherence. JAMA. 2015;313(14):1443-1450.
14. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2015.
1. January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society [published correction appears in J Am Coll Cardiol. 2014;64(21):2305-2307. J Am Coll Cardiol. 2014;64(21):e1-e76.
2. Reiffel JA. New versus traditional approaches to oral anticoagulation in patients with atrial fibrillation. Am J Med. 2014;127(4):e15.
3. Locke C, Ravnan SL, Patel R, Uchizono JA. Reduction in warfarin adverse events requiring patient hospitalization after implementation of pharmacist-managed anticoagulation service. Pharmacotherapy. 2005;25(5):685-689.
4. Poon IO, Lal L, Brown EN, Braun UK. The impact of pharmacist-managed oral anticoagulation therapy in older veterans. J Clin Pharm Ther. 2007;32(1):21-29.
5. Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care. Arch Intern Med. 1998;158(15):1641-1647.
6. The Joint Commission. National patient safety goals. https://www.jointcommission.org/as sets/1/6/2017_NPSG_HAP_ER.pdf. Published 2016. Accessed December 6, 2016.
7. Department of Veterans Affairs Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis): Criteria for Use for Stroke Prevention in nonvalvular atrial fibrillation (AF) and Edoxaban (SAVAYSA). http://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse/Anticoagulants_Direct_Oral_DOACs_CFU_and_Algorithm_for_Nonvalvular_Atrial_Fibrillation_Sep_2016.pdf. Updated September 2016. Accessed December 6, 2016.
8. Witt DM, Sadler MA, Shanahan RL, Mazzoli G, Tillman DJ. Effect of a centralized clinical pharmacy anticoagulation service on the outcomes of anticoagulation therapy. Chest. 2005;127(5):1515-1522.
9. Chan LL, Crumpler WL, Jacobson AK. Implementation of pharmacist-managed anticoagulation in patients receiving newer anticoagulants. Am J Health Syst Pharm. 2013;70(15):1285-1286, 1288.
10. Lee PY, Han SY, Miyahara RK. Adherence and outcomes of patients treated with dabigatran: pharmacist-managed anticoagulation clinic versus usual care. Am J Health Syst Pharm. 2013;70(13):1154-1161.
11. Shore S, Carey EP, Turakhia MP, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the Veterans Health Administration. Am Heart J. 2014;167(6):810-817.
12. Martin BC, Wiley-Exley EK, Richards S, Domino ME, Carey TS, Sleath BL. Contrasting measures of adherence with simple drug use, medication switching and therapeutic duplication. Ann Pharmacother. 2009;43(1):36-44.
13. Shore S, Ho PM, Lambert-Kerzner A, et al. Site-level variation in and practices associated with dabigatran adherence. JAMA. 2015;313(14):1443-1450.
14. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2015.