User login
SAN DIEGO – Adding daratumumab (D) to lenalidomide and dexamethasone (Rd) significantly improved outcomes in relapsed and refractory multiple myeloma, even when patients had previously received lenalidomide, were refractory to bortezomib, or had high-risk tumor cytogenetics, based on updated analyses from the multicenter, randomized, phase III, open-label POLLUX trial.
The findings underscore the “significant benefit of combining daratumumab with lenalidomide and dexamethasone for relapsed or refractory multiple myeloma,” said lead investigator Philippe Moreau, MD, of University Hospital Hotel-Dieu in Nantes, France.
Among a large subgroup of 524 POLLUX patients who had received one to three prior lines of therapy, estimated median progression-free survival (PFS) has not been reached in the daratumumab, lenalidomide, and dexamethasone (DRd) arm, versus 18.4 months in the lenalidomide and dexamethasone (Rd) arm (hazard ratio, 0.36; 95% CI: 0.26 to 0.49; P less than .0001), Dr. Moreau said at the annual meeting of the American Society of Hematology.
That means adding daratumumab to Rd led to a 64% reduction in the risk of disease progression or death among patients with relapsed or refractory multiple myeloma, he noted. Fully 77% of DRd patients were alive without having progressed at 18 months, and responses “continued to deepen in the DRd group with longer follow-up,” he added.
Additional analyses supported the use of DRd in relapsed or refractory multiple myeloma, “irrespective of prior lenalidomide treatment or bortezomib refractoriness,” Dr. Moreau continued. He reported that DRd significantly improved PFS over Rd alone not only among 445 lenalidomide-naive patients (HR, 0.37; P less than .0001), but also among 91 lenalidomide-exposed patients (HR, 0.45; P = .04), 140 patients who were refractory to their most recent line of therapy (HR, 0.45; P = .001), and 99 bortezomib- refractory patients (HR 0.51; P = .02).
Daratumumab (Darzalex), a human CD38 IgG1k monoclonal antibody, was first approved as monotherapy for multiple myeloma in patients who had received at least three prior lines of therapy or had double-refractory disease. In 2016, results from the twin POLLUX and CASTOR studies won daratumumab a Food and Drug Administration breakthrough designation status for use with Rd in patients who had received at least one prior line.
The POLLUX trial included 569 patients with multiple myeloma who had received a median of 1 and up to 11 prior lines of therapy. Patients were randomized to either Rd alone or to Rd plus intravenous daratumumab (16 mg/kg) once a week during the first two 28-day treatment cycles, every 2 weeks during cycles 3-6, and once only on day 1 of subsequent cycles.
POLLUX patients were fairly heavily pretreated, Dr. Moreau noted. Thirteen percent had received three prior lines of therapy, 86% had received a proteasome inhibitor, 18% had received lenalidomide, 21% were refractory to bortezomib, and 28% were refractory to their most recent line of therapy.
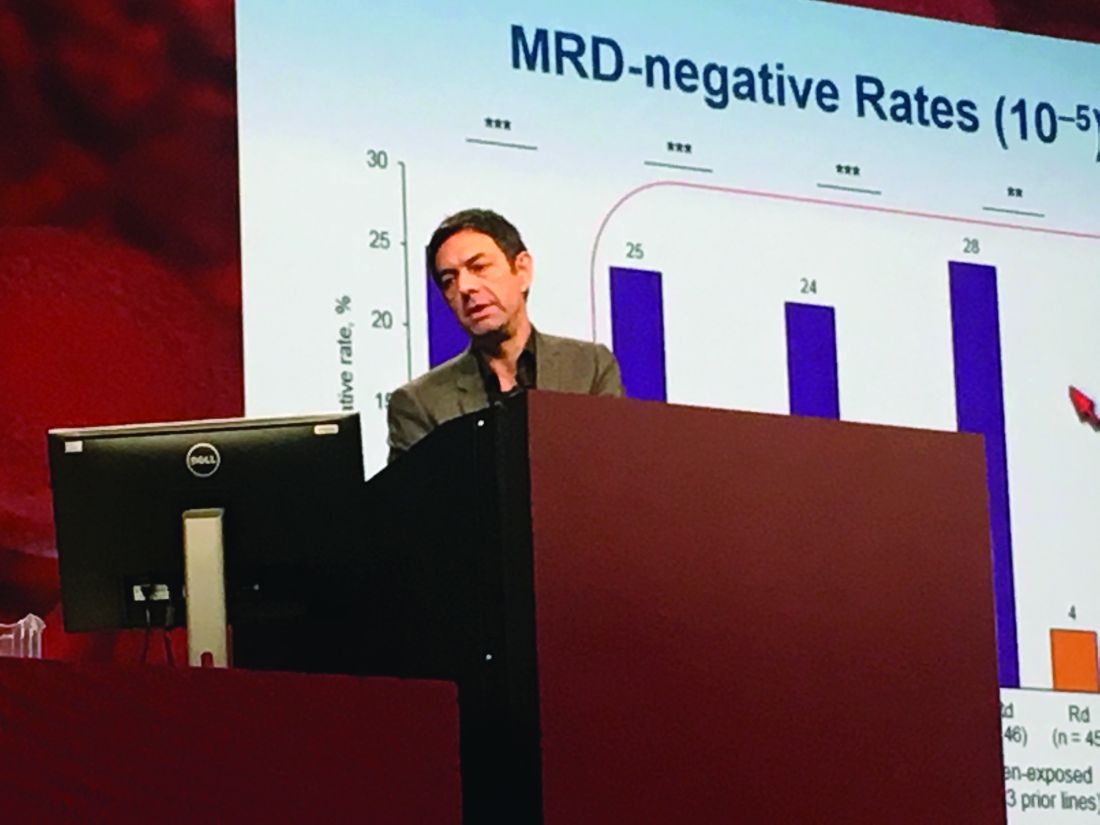
Researchers performed “stringent, unbiased” assessments of minimal residual disease (MRD) negativity not only when a complete response was suspected, but also 3 and 6 months later, Dr. Moreau said. He emphasized that rates of MRD negativity in lenalidomide-exposed, bortezomib-refractory subgroups in POLLUX almost exactly matched those in the intent-to-treat population (25% on DRd vs. 6% on Rd; P less than .0001).
A total of 17% of DRd patients and 25% of Rd patients had high-risk cytogenetic profiles, and DRd performed well in these individuals, Dr. Moreau reported. Fully 85% of all evaluable high-risk patients had at least a partial response to DRd, and 33% had a complete response, versus only 67% and 6% of high-risk Rd patients, respectively. Among patients with standard-risk cytogenetics, rates of best overall response were 95% on DRd and 82% on Rd, and rates of complete response were 52% on DRd and 24% on Rd.
POLLUX yielded no new safety signals for DRd, Dr. Moreau said. Rates of primary and secondary malignancies were less than 2%. Neutropenia, the most common adverse effect, was managed by interrupting treatment, reducing the dose of lenalidomide, and administering growth factor.
Janssen Research & Development funded the study. Dr. Moreau had no relevant financial disclosures.
SAN DIEGO – Adding daratumumab (D) to lenalidomide and dexamethasone (Rd) significantly improved outcomes in relapsed and refractory multiple myeloma, even when patients had previously received lenalidomide, were refractory to bortezomib, or had high-risk tumor cytogenetics, based on updated analyses from the multicenter, randomized, phase III, open-label POLLUX trial.
The findings underscore the “significant benefit of combining daratumumab with lenalidomide and dexamethasone for relapsed or refractory multiple myeloma,” said lead investigator Philippe Moreau, MD, of University Hospital Hotel-Dieu in Nantes, France.
Among a large subgroup of 524 POLLUX patients who had received one to three prior lines of therapy, estimated median progression-free survival (PFS) has not been reached in the daratumumab, lenalidomide, and dexamethasone (DRd) arm, versus 18.4 months in the lenalidomide and dexamethasone (Rd) arm (hazard ratio, 0.36; 95% CI: 0.26 to 0.49; P less than .0001), Dr. Moreau said at the annual meeting of the American Society of Hematology.
That means adding daratumumab to Rd led to a 64% reduction in the risk of disease progression or death among patients with relapsed or refractory multiple myeloma, he noted. Fully 77% of DRd patients were alive without having progressed at 18 months, and responses “continued to deepen in the DRd group with longer follow-up,” he added.
Additional analyses supported the use of DRd in relapsed or refractory multiple myeloma, “irrespective of prior lenalidomide treatment or bortezomib refractoriness,” Dr. Moreau continued. He reported that DRd significantly improved PFS over Rd alone not only among 445 lenalidomide-naive patients (HR, 0.37; P less than .0001), but also among 91 lenalidomide-exposed patients (HR, 0.45; P = .04), 140 patients who were refractory to their most recent line of therapy (HR, 0.45; P = .001), and 99 bortezomib- refractory patients (HR 0.51; P = .02).
Daratumumab (Darzalex), a human CD38 IgG1k monoclonal antibody, was first approved as monotherapy for multiple myeloma in patients who had received at least three prior lines of therapy or had double-refractory disease. In 2016, results from the twin POLLUX and CASTOR studies won daratumumab a Food and Drug Administration breakthrough designation status for use with Rd in patients who had received at least one prior line.
The POLLUX trial included 569 patients with multiple myeloma who had received a median of 1 and up to 11 prior lines of therapy. Patients were randomized to either Rd alone or to Rd plus intravenous daratumumab (16 mg/kg) once a week during the first two 28-day treatment cycles, every 2 weeks during cycles 3-6, and once only on day 1 of subsequent cycles.
POLLUX patients were fairly heavily pretreated, Dr. Moreau noted. Thirteen percent had received three prior lines of therapy, 86% had received a proteasome inhibitor, 18% had received lenalidomide, 21% were refractory to bortezomib, and 28% were refractory to their most recent line of therapy.
Researchers performed “stringent, unbiased” assessments of minimal residual disease (MRD) negativity not only when a complete response was suspected, but also 3 and 6 months later, Dr. Moreau said. He emphasized that rates of MRD negativity in lenalidomide-exposed, bortezomib-refractory subgroups in POLLUX almost exactly matched those in the intent-to-treat population (25% on DRd vs. 6% on Rd; P less than .0001).
A total of 17% of DRd patients and 25% of Rd patients had high-risk cytogenetic profiles, and DRd performed well in these individuals, Dr. Moreau reported. Fully 85% of all evaluable high-risk patients had at least a partial response to DRd, and 33% had a complete response, versus only 67% and 6% of high-risk Rd patients, respectively. Among patients with standard-risk cytogenetics, rates of best overall response were 95% on DRd and 82% on Rd, and rates of complete response were 52% on DRd and 24% on Rd.
POLLUX yielded no new safety signals for DRd, Dr. Moreau said. Rates of primary and secondary malignancies were less than 2%. Neutropenia, the most common adverse effect, was managed by interrupting treatment, reducing the dose of lenalidomide, and administering growth factor.
Janssen Research & Development funded the study. Dr. Moreau had no relevant financial disclosures.
SAN DIEGO – Adding daratumumab (D) to lenalidomide and dexamethasone (Rd) significantly improved outcomes in relapsed and refractory multiple myeloma, even when patients had previously received lenalidomide, were refractory to bortezomib, or had high-risk tumor cytogenetics, based on updated analyses from the multicenter, randomized, phase III, open-label POLLUX trial.
The findings underscore the “significant benefit of combining daratumumab with lenalidomide and dexamethasone for relapsed or refractory multiple myeloma,” said lead investigator Philippe Moreau, MD, of University Hospital Hotel-Dieu in Nantes, France.
Among a large subgroup of 524 POLLUX patients who had received one to three prior lines of therapy, estimated median progression-free survival (PFS) has not been reached in the daratumumab, lenalidomide, and dexamethasone (DRd) arm, versus 18.4 months in the lenalidomide and dexamethasone (Rd) arm (hazard ratio, 0.36; 95% CI: 0.26 to 0.49; P less than .0001), Dr. Moreau said at the annual meeting of the American Society of Hematology.
That means adding daratumumab to Rd led to a 64% reduction in the risk of disease progression or death among patients with relapsed or refractory multiple myeloma, he noted. Fully 77% of DRd patients were alive without having progressed at 18 months, and responses “continued to deepen in the DRd group with longer follow-up,” he added.
Additional analyses supported the use of DRd in relapsed or refractory multiple myeloma, “irrespective of prior lenalidomide treatment or bortezomib refractoriness,” Dr. Moreau continued. He reported that DRd significantly improved PFS over Rd alone not only among 445 lenalidomide-naive patients (HR, 0.37; P less than .0001), but also among 91 lenalidomide-exposed patients (HR, 0.45; P = .04), 140 patients who were refractory to their most recent line of therapy (HR, 0.45; P = .001), and 99 bortezomib- refractory patients (HR 0.51; P = .02).
Daratumumab (Darzalex), a human CD38 IgG1k monoclonal antibody, was first approved as monotherapy for multiple myeloma in patients who had received at least three prior lines of therapy or had double-refractory disease. In 2016, results from the twin POLLUX and CASTOR studies won daratumumab a Food and Drug Administration breakthrough designation status for use with Rd in patients who had received at least one prior line.
The POLLUX trial included 569 patients with multiple myeloma who had received a median of 1 and up to 11 prior lines of therapy. Patients were randomized to either Rd alone or to Rd plus intravenous daratumumab (16 mg/kg) once a week during the first two 28-day treatment cycles, every 2 weeks during cycles 3-6, and once only on day 1 of subsequent cycles.
POLLUX patients were fairly heavily pretreated, Dr. Moreau noted. Thirteen percent had received three prior lines of therapy, 86% had received a proteasome inhibitor, 18% had received lenalidomide, 21% were refractory to bortezomib, and 28% were refractory to their most recent line of therapy.
Researchers performed “stringent, unbiased” assessments of minimal residual disease (MRD) negativity not only when a complete response was suspected, but also 3 and 6 months later, Dr. Moreau said. He emphasized that rates of MRD negativity in lenalidomide-exposed, bortezomib-refractory subgroups in POLLUX almost exactly matched those in the intent-to-treat population (25% on DRd vs. 6% on Rd; P less than .0001).
A total of 17% of DRd patients and 25% of Rd patients had high-risk cytogenetic profiles, and DRd performed well in these individuals, Dr. Moreau reported. Fully 85% of all evaluable high-risk patients had at least a partial response to DRd, and 33% had a complete response, versus only 67% and 6% of high-risk Rd patients, respectively. Among patients with standard-risk cytogenetics, rates of best overall response were 95% on DRd and 82% on Rd, and rates of complete response were 52% on DRd and 24% on Rd.
POLLUX yielded no new safety signals for DRd, Dr. Moreau said. Rates of primary and secondary malignancies were less than 2%. Neutropenia, the most common adverse effect, was managed by interrupting treatment, reducing the dose of lenalidomide, and administering growth factor.
Janssen Research & Development funded the study. Dr. Moreau had no relevant financial disclosures.
AT ASH 2016
Key clinical point: Adding daratumumab (D) to lenalidomide and dexamethasone (Rd) significantly improved outcomes in patients with relapsed and refractory multiple myeloma, regardless of factors such as bortezomib refractoriness, lenalidomide exposure, or high-risk tumor cytogenetics.
Major finding: DRd significantly improved PFS over Rd not only among 445 lenalidomide-naive patients (HR, 0.37; P less than .0001), but also among 91 lenalidomide-exposed patients (HR, 0.45; P = .04), 140 patients who were refractory to their most recent line of therapy (HR, 0.45; P = .001), and 99 bortezomib-refractory patients (HR 0.51; P = .02).
Data source: POLLUX, a multicenter, randomized, phase III, open-label trial of 569 patients with multiple myeloma who had received one or more previous lines of therapy.
Disclosures: Janssen Research & Development funded the study. Dr. Moreau had no relevant financial disclosures.