User login
Patients with newly diagnosed multiple myeloma or smoldering multiple myeloma experienced good responses and few severe adverse events from carfilzomib-lenalidomide-dexamethasone with lenalidomide extension (CRd-R), according to a report published online in JAMA Oncology.
The regimen was not associated with peripheral neuropathy of grade 3 or above, and both groups had high rates of minimal residual disease negativity, which has been shown to be associated with improved progression-free survival and overall survival in newly diagnosed patients treated with CRd.
However, the three tools used for measuring patient response differed in their abilities to detect minimal residual disease, the researchers reported.
“Given the high degree of CR [complete response] rates achievable by three-drug combination regimens, there is an increased need for clinical trials to detect MRD [minimal residual disease] beyond traditional methods and characterize optimal MRD technique,” wrote Dr. Neha Korde of the myeloma service at Memorial Sloan Kettering Cancer Center, New York, and colleagues.
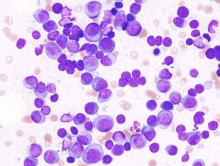
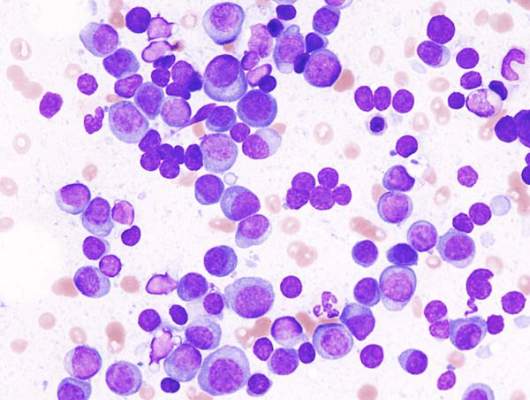
The researchers used three measures to assess minimal residual disease: multiparametric flow cytometry, next-generation sequencing, and fluorodeoxyglucose positron-emission tomography/computed tomography.
Based on multiparametric flow cytometry, minimal residual disease was not detected in 62% (28/45) of the patients with newly diagnosed disease and in 92% (11/12) of those with smoldering multiple myeloma, (JAMA Oncol. 2015 July 2 [doi:10.1001/jamaoncol.2015.2010]). Based on flow cytometry, 98% negativity for minimal residual disease was seen in patients with at least nCR. Based on next-generation sequencing, an additional 30% of patients were positive for minimal residual disease, highlighting the potential increased sensitivity of that assay, the researcher said.
In patients with newly diagnosed disease, 98% achieved at least a partial response, 89% had at least a very good partial response, 62% had at least a near complete response, and 56% had at least a complete response or a stringent complete response. Responses improved with more therapy; the median time to CR was 5 (2-17) cycles, and six patients reached CR during the lenalidomide extension. The median duration of response was not reached.
All of the patients with high-risk smoldering multiple myeloma achieved at least a complete response, with median time of 6 (2-20) cycles to CR.
In a 2-year study, 45 patients with newly diagnosed multiple myeloma received a treatment regimen that included eight 28-day cycles of CRd, followed by 24 cycles of lenalidomide extended dosing for those with stable disease. Promising early results led to a pilot study with the same regimen in 12 patients with high-risk SMM, a condition that leads to symptomatic disease within a median of 2 years.
None of the patients with newly diagnosed multiple myeloma had peripheral neuropathy of grade 3 or above, the primary endpoint. Grade 1 peripheral neuropathy affected 33% of patients, and grade 2 was experienced by 9%. Lymphopenia and electrolyte or metabolism abnormalities were the most common adverse events. Grade 3 or 4 cardiac events included two patients with congestive heart failure and three patients with hypertension.
Patients with smoldering multiple myeloma had similar rates of adverse events.
In this pilot study, the CRd combination was well tolerated and yielded deep responses across prognostic groups. However, morphological complete response, in this scenario, was insufficient to characterize tumor reduction.
“With the introduction of highly effective drug combinations, traditional response criteria become less valid because these do not sufficiently assess the deepness of response.” New approaches such as MRD and PET/CT negativity should be introduced to evaluate response in clinical trials and, ultimately, in clinical practice.
Patients with smoldering multiple myeloma, who have more than a 90% probability of disease progression within 2 years, may benefit from early treatment that is effective and does not expose them to an excessive risk of toxicity.
Dr. Pieter Sonneveld is head of the department of hematology at the Erasmus MC Cancer Institute, Rotterdam, the Netherlands. Dr. Sonneveld reported receiving research funding from Celgene, Onyx/Amgen, Janssen, and Karyopharm.
In this pilot study, the CRd combination was well tolerated and yielded deep responses across prognostic groups. However, morphological complete response, in this scenario, was insufficient to characterize tumor reduction.
“With the introduction of highly effective drug combinations, traditional response criteria become less valid because these do not sufficiently assess the deepness of response.” New approaches such as MRD and PET/CT negativity should be introduced to evaluate response in clinical trials and, ultimately, in clinical practice.
Patients with smoldering multiple myeloma, who have more than a 90% probability of disease progression within 2 years, may benefit from early treatment that is effective and does not expose them to an excessive risk of toxicity.
Dr. Pieter Sonneveld is head of the department of hematology at the Erasmus MC Cancer Institute, Rotterdam, the Netherlands. Dr. Sonneveld reported receiving research funding from Celgene, Onyx/Amgen, Janssen, and Karyopharm.
In this pilot study, the CRd combination was well tolerated and yielded deep responses across prognostic groups. However, morphological complete response, in this scenario, was insufficient to characterize tumor reduction.
“With the introduction of highly effective drug combinations, traditional response criteria become less valid because these do not sufficiently assess the deepness of response.” New approaches such as MRD and PET/CT negativity should be introduced to evaluate response in clinical trials and, ultimately, in clinical practice.
Patients with smoldering multiple myeloma, who have more than a 90% probability of disease progression within 2 years, may benefit from early treatment that is effective and does not expose them to an excessive risk of toxicity.
Dr. Pieter Sonneveld is head of the department of hematology at the Erasmus MC Cancer Institute, Rotterdam, the Netherlands. Dr. Sonneveld reported receiving research funding from Celgene, Onyx/Amgen, Janssen, and Karyopharm.
Patients with newly diagnosed multiple myeloma or smoldering multiple myeloma experienced good responses and few severe adverse events from carfilzomib-lenalidomide-dexamethasone with lenalidomide extension (CRd-R), according to a report published online in JAMA Oncology.
The regimen was not associated with peripheral neuropathy of grade 3 or above, and both groups had high rates of minimal residual disease negativity, which has been shown to be associated with improved progression-free survival and overall survival in newly diagnosed patients treated with CRd.
However, the three tools used for measuring patient response differed in their abilities to detect minimal residual disease, the researchers reported.
“Given the high degree of CR [complete response] rates achievable by three-drug combination regimens, there is an increased need for clinical trials to detect MRD [minimal residual disease] beyond traditional methods and characterize optimal MRD technique,” wrote Dr. Neha Korde of the myeloma service at Memorial Sloan Kettering Cancer Center, New York, and colleagues.
The researchers used three measures to assess minimal residual disease: multiparametric flow cytometry, next-generation sequencing, and fluorodeoxyglucose positron-emission tomography/computed tomography.
Based on multiparametric flow cytometry, minimal residual disease was not detected in 62% (28/45) of the patients with newly diagnosed disease and in 92% (11/12) of those with smoldering multiple myeloma, (JAMA Oncol. 2015 July 2 [doi:10.1001/jamaoncol.2015.2010]). Based on flow cytometry, 98% negativity for minimal residual disease was seen in patients with at least nCR. Based on next-generation sequencing, an additional 30% of patients were positive for minimal residual disease, highlighting the potential increased sensitivity of that assay, the researcher said.
In patients with newly diagnosed disease, 98% achieved at least a partial response, 89% had at least a very good partial response, 62% had at least a near complete response, and 56% had at least a complete response or a stringent complete response. Responses improved with more therapy; the median time to CR was 5 (2-17) cycles, and six patients reached CR during the lenalidomide extension. The median duration of response was not reached.
All of the patients with high-risk smoldering multiple myeloma achieved at least a complete response, with median time of 6 (2-20) cycles to CR.
In a 2-year study, 45 patients with newly diagnosed multiple myeloma received a treatment regimen that included eight 28-day cycles of CRd, followed by 24 cycles of lenalidomide extended dosing for those with stable disease. Promising early results led to a pilot study with the same regimen in 12 patients with high-risk SMM, a condition that leads to symptomatic disease within a median of 2 years.
None of the patients with newly diagnosed multiple myeloma had peripheral neuropathy of grade 3 or above, the primary endpoint. Grade 1 peripheral neuropathy affected 33% of patients, and grade 2 was experienced by 9%. Lymphopenia and electrolyte or metabolism abnormalities were the most common adverse events. Grade 3 or 4 cardiac events included two patients with congestive heart failure and three patients with hypertension.
Patients with smoldering multiple myeloma had similar rates of adverse events.
Patients with newly diagnosed multiple myeloma or smoldering multiple myeloma experienced good responses and few severe adverse events from carfilzomib-lenalidomide-dexamethasone with lenalidomide extension (CRd-R), according to a report published online in JAMA Oncology.
The regimen was not associated with peripheral neuropathy of grade 3 or above, and both groups had high rates of minimal residual disease negativity, which has been shown to be associated with improved progression-free survival and overall survival in newly diagnosed patients treated with CRd.
However, the three tools used for measuring patient response differed in their abilities to detect minimal residual disease, the researchers reported.
“Given the high degree of CR [complete response] rates achievable by three-drug combination regimens, there is an increased need for clinical trials to detect MRD [minimal residual disease] beyond traditional methods and characterize optimal MRD technique,” wrote Dr. Neha Korde of the myeloma service at Memorial Sloan Kettering Cancer Center, New York, and colleagues.
The researchers used three measures to assess minimal residual disease: multiparametric flow cytometry, next-generation sequencing, and fluorodeoxyglucose positron-emission tomography/computed tomography.
Based on multiparametric flow cytometry, minimal residual disease was not detected in 62% (28/45) of the patients with newly diagnosed disease and in 92% (11/12) of those with smoldering multiple myeloma, (JAMA Oncol. 2015 July 2 [doi:10.1001/jamaoncol.2015.2010]). Based on flow cytometry, 98% negativity for minimal residual disease was seen in patients with at least nCR. Based on next-generation sequencing, an additional 30% of patients were positive for minimal residual disease, highlighting the potential increased sensitivity of that assay, the researcher said.
In patients with newly diagnosed disease, 98% achieved at least a partial response, 89% had at least a very good partial response, 62% had at least a near complete response, and 56% had at least a complete response or a stringent complete response. Responses improved with more therapy; the median time to CR was 5 (2-17) cycles, and six patients reached CR during the lenalidomide extension. The median duration of response was not reached.
All of the patients with high-risk smoldering multiple myeloma achieved at least a complete response, with median time of 6 (2-20) cycles to CR.
In a 2-year study, 45 patients with newly diagnosed multiple myeloma received a treatment regimen that included eight 28-day cycles of CRd, followed by 24 cycles of lenalidomide extended dosing for those with stable disease. Promising early results led to a pilot study with the same regimen in 12 patients with high-risk SMM, a condition that leads to symptomatic disease within a median of 2 years.
None of the patients with newly diagnosed multiple myeloma had peripheral neuropathy of grade 3 or above, the primary endpoint. Grade 1 peripheral neuropathy affected 33% of patients, and grade 2 was experienced by 9%. Lymphopenia and electrolyte or metabolism abnormalities were the most common adverse events. Grade 3 or 4 cardiac events included two patients with congestive heart failure and three patients with hypertension.
Patients with smoldering multiple myeloma had similar rates of adverse events.
FROM JAMA ONCOLOGY
Key clinical point: Patients with newly diagnosed multiple myeloma or smoldering multiple myeloma had high rates of minimal residual disease negativity and none experienced grade 3 or 4 peripheral neuropathy from carfilzomib-lenalidomide-dexamethasone with lenalidomide extension (CRd-R).
Major finding: Based on multiparametric flow cytometry, minimal residual disease was not detected in 62% (28/45) of the patients with newly diagnosed disease and in 92% (11/12) of those with smoldering multiple myeloma.
Data source: A 2-year pilot study of 45 patients with newly diagnosed multiple myeloma and 12 patients with smoldering multiple myeloma.
Disclosures: The study was partially supported by Onyx and Celgene. Dr. Korde reported consulting for MedScape. Her coauthors reported ties to several industry sources.