User login
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
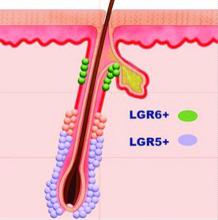
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.