User login
Antineutrophil cytoplasmic antibody (ANCA) detection is a valuable tool for diagnosing small-vessel vasculitis,1 but measuring and interpreting ANCA levels is an inexact science. There is no single perfect ANCA test, and even a perfect test would not provide definitive clinical answers. The diagnostic utility of ANCA testing depends on the methodologic accuracy of the test and the appropriate ordering of testing in the right clinical setting. This article examines three important questions about this technology:
- What is the best ANCA test methodology?
- What is the prognostic value of serial ANCA testing?
- What is the clinical implication of ANCA type?
WHAT IS THE BEST ANCA TEST METHODOLOGY?
The diagnostic utility of ANCA testing depends on both the methodologic accuracy of the test and the appropriate ordering of tests. Methodologic accuracy comprises the analytic sensitivity and specificity of the test. Analytic sensitivity refers to the accurate identification of the presence of ANCA, and analytic specificity refers to measurement of only the entity in question (ANCA), not confounded by the presence of other entities (antibodies).
Equally as important as analytic accuracy is the appropriate ordering of the tests in the right clinical setting. Using a test that is sensitive to the presence of a specific ANCA type accurately identifies the presence of either proteinase-3 (PR3)- or myeloperoxidase (MPO)-ANCA. Once obtained, test results must be evaluated in terms of their relationship to the diagnosis being considered. If the tests are deemed diagnostically useful based on the results, the data can be used to assess the positive and negative predictive value of the tests.
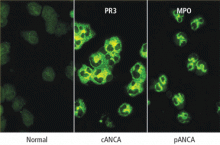
Immunofluorescence or antigen-specific testing—or both?
A definitive diagnosis is more likely if an immunofluorescence staining pattern of cANCA is paired with the antigen specificity of PR3-ANCA, for example, or a perinuclear immunofluorescence pattern (pANCA) is paired with a positive MPO-ANCA. When positive test pairings have been obtained and the patient’s antigen ANCA reactivity is known, subsequent serial ANCA testing with an antigen-specific assay alone may be indicated, because the ANCA types of patients with vasculitis are unlikely to switch between PR3 and MPO during the course of their disease. If matching pairings are not obtained, the diagnostic utility of the tests remains unconfirmed.
Antigen type (PR3 or MPO) is determined through antigen-specific methods that include solid-phase assays and other methods of bringing the specific antigen in contact with the specific antibody in question. There are two categories of solid-phase assays: the enzyme-linked immunoabsorbent assay (ELISA) and the capture ELISA. In the ELISA methodology, the antigen is directly coated to a plastic plate; in the capture ELISA, an anchor, usually a monoclonal antibody or combination of antibodies, captures the target antigen on the plate. In both ELISA and capture ELISA assays, ANCA contained in the serum sample subjected to testing bind to the immobilized antigen. The amount of ANCA bound to the antigen can then be detected by a secondary antibody that is conjugated with an enzyme that can elicit a color reaction. The intensity of the color reaction is proportional to the amount of ANCA bound to the immobilized antigen.
The ELISA methodology tends to trade off analytic sensitivity for specificity, since the antigen purification process (which allows the ELISA system to increase its specificity) can cause conformational changes to the antigen being bound to the plate. This, in turn, causes a loss of some recognition of the conformationally sensitive ANCA.
In capture ELISA, a specific antibody captures the antigen; this stabilizes the conformation, boosts the analytic sensitivity, and allows a gentler purification process because it only captures the antigen in question and then binds it to the plate. This process decreases false-positive test results caused by residual contaminants in the antigen preparation. Analytic sensitivity issues may come into play if the anchoring monoclonal antibody competes for the epitope on the antigen being recognized by the serum antibody in question (ANCA), causing occasional false-negative results.
Another method now applied to commercial ANCA testing involves bead-based multiplex assays. These assays are based on principles similar to the ELISA or capture ELISA methods. In multiplex microsphere technology, the purified antigen is bound to a polystyrene microsphere instead of a plate. The microsphere is then presented to the antibody in question. The bead is then introduced to a secondary antibody labeled with a fluorescent marker (instead of an enzyme) for detection of the antibody. One advantage of this system is that various beads containing different antigens can be introduced to the same serum sample, and then different color reactions can be measured for each bead. Because only one antigen is bound to each microsphere (eg, PR3-ANCA, MPOANCA or other specific autoantibodies), only specific antibodies will react to each bead of a specific color. If there is no MPO antibody in the sample, there will be no reaction against the MPO antigen bead; however, if PR3-ANCA is present in the sample, it would react with the PR3 antigen beads. Using this methodology, a single serum sample can be tested for a multitude of autoantibodies at the same time (see “Interpreting ANCA results: Accurate tests, appropriate orders,”2–10 above).

WHAT IS THE PROGNOSTIC VALUE OF SERIAL ANCA TESTING?
Persistent changes in ANCA levels in relapsing disease may have some value in predicting outcome. The issues to consider include the methodology used to determine serial ANCA levels, correlations between ANCA and disease activity, and the use of ANCA changes to guide treatment.
Does methodology matter when determining serial ANCA levels?
Methodology in serial ANCA testing is probably unimportant as long as the same method is used serially. Analysis of large groups of ANCA-positive patients show a statistically highly significant correlation among results obtained with different detection methods, including immunofluorescence, direct ELISA, or capture ELISA. However, at the individual patient level there is some variability.
Do ANCA levels correlate with disease activity?
In a prospective study, serial ANCA samples obtained during the Wegener’s Granulomatosis Etanercept Trial (WGET)11 were processed in the same manner (collected every 3 months, mean follow-up of 22 months, uniform handling of samples). All samples were analyzed by capture ELISA, and disease activity was measured by the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG). The results indicated that an increase in PR3-ANCA levels was not a significant predictor of relapse. The frequency of a relapse within 1 year of an increase in PR3-ANCA levels was found to be approximately 50%,11 a result similar to that reported in several smaller studies of different design and methodology.
Should ANCA changes guide treatment?
The available data regarding serial ANCA testing are limited mostly to PR3-ANCA. Serial ANCA testing has limited value as a guide to treatment and, in general, changes in ANCA levels alone should not be used to guide treatment decisions. In new patients without documented serial ANCA level associations, an increase in PR3-ANCA levels has no reliable predictive value. The existing literature suggests that this lack of association is not dependent on the method used for ANCA detection. For individual patients in whom long-term serial ANCA testing has been performed and a relationship between PR3-ANCA levels and disease activity has been established, serial ANCA testing can have some predictive value and can be used to guide treatment. For example, when remission is achieved by depleting B cells in patients with chronically relapsing GPA, ANCA levels usually go down. After B-cell reconstitution, the ANCA level rises in most patients, and this rise is usually associated with a flare shortly thereafter. A flare can be preempted when this pattern is determined in a specific patient, and preemptive treatment is applied accordingly.12
WHAT IS THE IMPLICATION OF ANCA TYPE?
Available reports consistently suggest that PR3-ANCA is associated with a higher mortality than MPO-ANCA (relative risk [RR], 3.78),13 and a higher relapse rate.14,15 A more rapid loss of renal function among patients with glomerulonephritis and PR3-ANCA than those with MPO-ANCA has also been reported.16 Using remission as the starting point, the number of days from complete remission to first disease flare was plotted for patients with MPO- versus PR3-ANCA in an analysis of long-term data from the Rituximab in ANCA-Associated Vasculitis (RAVE) trial.17 The resulting curve demonstrated a divergence in the probability of remaining in remission, confirming that remission maintenance is clearly greater in patients with MPO-ANCA than in patients with PR3-ANCA.
The primary end point of the RAVE trial was remission of disease without the use of prednisone at 6 months. There was little difference in end point achieved based on comparison of diagnosis (microscopic polyangiitis or granulomatosis) or treatment arms (rituximab versus cyclophosphamide); however, an analysis of end point data separating the patients by ANCA type showed that the treatment response to rituximab was superior to that of cyclophosphamide among patients with PR3-ANCA, whereas in patients with MPO-ANCA, there was little difference in response associated with either treatment. Regarding the likelihood of attaining an ANCA-negative status after 6 months, again MPO-ANCA patients showed no difference in frequency on either treatment. Among PR3-ANCA–positive patients, 50% in the rituximab arm attained ANCA-negative status compared with only 17% in the cyclophosphamide arm.17
SUMMARY
Diagnostic utility of ANCA testing depends on the methodology and clinical setting. Only cANCA/PR3-ANCA and pANCA/MPO-ANCA pairings have positive predictive value for diagnosis of small-vessel vasculitis. Mismatches in results, findings of human neutrophil elastase–ANCA, or identification of multiple positive antigens should be considered in cases of cocaine or drug use.
The clinical utility of serial ANCA testing is unconfirmed. Good data currently exist only for PR3-ANCA, and different drugs may affect ANCA levels in different ways. ANCA type is significant in that PR3-ANCA portends a higher relapse rate and poorer patient outcomes compared with MPO-ANCA.
- Russell KA, Wiegert E, Schroeder DR, Homburger HA, Specks U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin Immunol 2002; 103:196–203.
- Langford CA. The diagnostic utility of c-ANCA in Wegener’s granulomatosis. Cleve Clin J Med 1998; 65:135–140.
- Trimarchi M, Gregorini G, Facchetti F, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine 2001; 80:391–404.
- Wiesner O, Russell KA, Lee AS, et al. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum 2004; 50:2954–2965.
- Peikert T, Finkielman JD, Hummel AM, et al. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum 2008; 58:1546–1551.
- Knowles L, Buxton JA, Skuridina N, et al. Levamisole tainted cocaine causing severe neutropenia in Alberta and British Columbia. Harm Reduct J 2009; 6 (Nov 17):30. doi: 10.1186/1477-7517-6-30.
- Zhu NY, LeGatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med 2009; 150:287–289.
- Bradford M, Rosenberg B, Moreno J, Dumyati G. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med 2010; 152:758–759.
- Waller JM, Feramisco JD, Alberta-Wszolek L, McCalmont TH, Fox LP. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol 2010; 63:530–535. doi: 10.1016/j.jaad.2010.01.055
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant [published online ahead of print July 28, 2010]. Clin Pharmacol Ther 2010; 88:408–411. doi: 10.1038/clpt.2010.156
- Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med 2007; 147:611–619.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
- Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ; the Glomerular Disease Collaborative Network. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7:23–32.
- Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Franssen CFM, Gans ROB, Arends B, et al. Differences between anti-myeloperoxidase- and anti-proteinase 3-associated renal disease. Kidney Int 1995; 47:193–199.
- Stone JH, Merkel PA, Spiera R, et al; for the RAVE–ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
Antineutrophil cytoplasmic antibody (ANCA) detection is a valuable tool for diagnosing small-vessel vasculitis,1 but measuring and interpreting ANCA levels is an inexact science. There is no single perfect ANCA test, and even a perfect test would not provide definitive clinical answers. The diagnostic utility of ANCA testing depends on the methodologic accuracy of the test and the appropriate ordering of testing in the right clinical setting. This article examines three important questions about this technology:
- What is the best ANCA test methodology?
- What is the prognostic value of serial ANCA testing?
- What is the clinical implication of ANCA type?
WHAT IS THE BEST ANCA TEST METHODOLOGY?
The diagnostic utility of ANCA testing depends on both the methodologic accuracy of the test and the appropriate ordering of tests. Methodologic accuracy comprises the analytic sensitivity and specificity of the test. Analytic sensitivity refers to the accurate identification of the presence of ANCA, and analytic specificity refers to measurement of only the entity in question (ANCA), not confounded by the presence of other entities (antibodies).
Equally as important as analytic accuracy is the appropriate ordering of the tests in the right clinical setting. Using a test that is sensitive to the presence of a specific ANCA type accurately identifies the presence of either proteinase-3 (PR3)- or myeloperoxidase (MPO)-ANCA. Once obtained, test results must be evaluated in terms of their relationship to the diagnosis being considered. If the tests are deemed diagnostically useful based on the results, the data can be used to assess the positive and negative predictive value of the tests.
Immunofluorescence or antigen-specific testing—or both?
A definitive diagnosis is more likely if an immunofluorescence staining pattern of cANCA is paired with the antigen specificity of PR3-ANCA, for example, or a perinuclear immunofluorescence pattern (pANCA) is paired with a positive MPO-ANCA. When positive test pairings have been obtained and the patient’s antigen ANCA reactivity is known, subsequent serial ANCA testing with an antigen-specific assay alone may be indicated, because the ANCA types of patients with vasculitis are unlikely to switch between PR3 and MPO during the course of their disease. If matching pairings are not obtained, the diagnostic utility of the tests remains unconfirmed.
Antigen type (PR3 or MPO) is determined through antigen-specific methods that include solid-phase assays and other methods of bringing the specific antigen in contact with the specific antibody in question. There are two categories of solid-phase assays: the enzyme-linked immunoabsorbent assay (ELISA) and the capture ELISA. In the ELISA methodology, the antigen is directly coated to a plastic plate; in the capture ELISA, an anchor, usually a monoclonal antibody or combination of antibodies, captures the target antigen on the plate. In both ELISA and capture ELISA assays, ANCA contained in the serum sample subjected to testing bind to the immobilized antigen. The amount of ANCA bound to the antigen can then be detected by a secondary antibody that is conjugated with an enzyme that can elicit a color reaction. The intensity of the color reaction is proportional to the amount of ANCA bound to the immobilized antigen.
The ELISA methodology tends to trade off analytic sensitivity for specificity, since the antigen purification process (which allows the ELISA system to increase its specificity) can cause conformational changes to the antigen being bound to the plate. This, in turn, causes a loss of some recognition of the conformationally sensitive ANCA.
In capture ELISA, a specific antibody captures the antigen; this stabilizes the conformation, boosts the analytic sensitivity, and allows a gentler purification process because it only captures the antigen in question and then binds it to the plate. This process decreases false-positive test results caused by residual contaminants in the antigen preparation. Analytic sensitivity issues may come into play if the anchoring monoclonal antibody competes for the epitope on the antigen being recognized by the serum antibody in question (ANCA), causing occasional false-negative results.
Another method now applied to commercial ANCA testing involves bead-based multiplex assays. These assays are based on principles similar to the ELISA or capture ELISA methods. In multiplex microsphere technology, the purified antigen is bound to a polystyrene microsphere instead of a plate. The microsphere is then presented to the antibody in question. The bead is then introduced to a secondary antibody labeled with a fluorescent marker (instead of an enzyme) for detection of the antibody. One advantage of this system is that various beads containing different antigens can be introduced to the same serum sample, and then different color reactions can be measured for each bead. Because only one antigen is bound to each microsphere (eg, PR3-ANCA, MPOANCA or other specific autoantibodies), only specific antibodies will react to each bead of a specific color. If there is no MPO antibody in the sample, there will be no reaction against the MPO antigen bead; however, if PR3-ANCA is present in the sample, it would react with the PR3 antigen beads. Using this methodology, a single serum sample can be tested for a multitude of autoantibodies at the same time (see “Interpreting ANCA results: Accurate tests, appropriate orders,”2–10 above).

WHAT IS THE PROGNOSTIC VALUE OF SERIAL ANCA TESTING?
Persistent changes in ANCA levels in relapsing disease may have some value in predicting outcome. The issues to consider include the methodology used to determine serial ANCA levels, correlations between ANCA and disease activity, and the use of ANCA changes to guide treatment.
Does methodology matter when determining serial ANCA levels?
Methodology in serial ANCA testing is probably unimportant as long as the same method is used serially. Analysis of large groups of ANCA-positive patients show a statistically highly significant correlation among results obtained with different detection methods, including immunofluorescence, direct ELISA, or capture ELISA. However, at the individual patient level there is some variability.
Do ANCA levels correlate with disease activity?
In a prospective study, serial ANCA samples obtained during the Wegener’s Granulomatosis Etanercept Trial (WGET)11 were processed in the same manner (collected every 3 months, mean follow-up of 22 months, uniform handling of samples). All samples were analyzed by capture ELISA, and disease activity was measured by the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG). The results indicated that an increase in PR3-ANCA levels was not a significant predictor of relapse. The frequency of a relapse within 1 year of an increase in PR3-ANCA levels was found to be approximately 50%,11 a result similar to that reported in several smaller studies of different design and methodology.
Should ANCA changes guide treatment?
The available data regarding serial ANCA testing are limited mostly to PR3-ANCA. Serial ANCA testing has limited value as a guide to treatment and, in general, changes in ANCA levels alone should not be used to guide treatment decisions. In new patients without documented serial ANCA level associations, an increase in PR3-ANCA levels has no reliable predictive value. The existing literature suggests that this lack of association is not dependent on the method used for ANCA detection. For individual patients in whom long-term serial ANCA testing has been performed and a relationship between PR3-ANCA levels and disease activity has been established, serial ANCA testing can have some predictive value and can be used to guide treatment. For example, when remission is achieved by depleting B cells in patients with chronically relapsing GPA, ANCA levels usually go down. After B-cell reconstitution, the ANCA level rises in most patients, and this rise is usually associated with a flare shortly thereafter. A flare can be preempted when this pattern is determined in a specific patient, and preemptive treatment is applied accordingly.12
WHAT IS THE IMPLICATION OF ANCA TYPE?
Available reports consistently suggest that PR3-ANCA is associated with a higher mortality than MPO-ANCA (relative risk [RR], 3.78),13 and a higher relapse rate.14,15 A more rapid loss of renal function among patients with glomerulonephritis and PR3-ANCA than those with MPO-ANCA has also been reported.16 Using remission as the starting point, the number of days from complete remission to first disease flare was plotted for patients with MPO- versus PR3-ANCA in an analysis of long-term data from the Rituximab in ANCA-Associated Vasculitis (RAVE) trial.17 The resulting curve demonstrated a divergence in the probability of remaining in remission, confirming that remission maintenance is clearly greater in patients with MPO-ANCA than in patients with PR3-ANCA.
The primary end point of the RAVE trial was remission of disease without the use of prednisone at 6 months. There was little difference in end point achieved based on comparison of diagnosis (microscopic polyangiitis or granulomatosis) or treatment arms (rituximab versus cyclophosphamide); however, an analysis of end point data separating the patients by ANCA type showed that the treatment response to rituximab was superior to that of cyclophosphamide among patients with PR3-ANCA, whereas in patients with MPO-ANCA, there was little difference in response associated with either treatment. Regarding the likelihood of attaining an ANCA-negative status after 6 months, again MPO-ANCA patients showed no difference in frequency on either treatment. Among PR3-ANCA–positive patients, 50% in the rituximab arm attained ANCA-negative status compared with only 17% in the cyclophosphamide arm.17
SUMMARY
Diagnostic utility of ANCA testing depends on the methodology and clinical setting. Only cANCA/PR3-ANCA and pANCA/MPO-ANCA pairings have positive predictive value for diagnosis of small-vessel vasculitis. Mismatches in results, findings of human neutrophil elastase–ANCA, or identification of multiple positive antigens should be considered in cases of cocaine or drug use.
The clinical utility of serial ANCA testing is unconfirmed. Good data currently exist only for PR3-ANCA, and different drugs may affect ANCA levels in different ways. ANCA type is significant in that PR3-ANCA portends a higher relapse rate and poorer patient outcomes compared with MPO-ANCA.
Antineutrophil cytoplasmic antibody (ANCA) detection is a valuable tool for diagnosing small-vessel vasculitis,1 but measuring and interpreting ANCA levels is an inexact science. There is no single perfect ANCA test, and even a perfect test would not provide definitive clinical answers. The diagnostic utility of ANCA testing depends on the methodologic accuracy of the test and the appropriate ordering of testing in the right clinical setting. This article examines three important questions about this technology:
- What is the best ANCA test methodology?
- What is the prognostic value of serial ANCA testing?
- What is the clinical implication of ANCA type?
WHAT IS THE BEST ANCA TEST METHODOLOGY?
The diagnostic utility of ANCA testing depends on both the methodologic accuracy of the test and the appropriate ordering of tests. Methodologic accuracy comprises the analytic sensitivity and specificity of the test. Analytic sensitivity refers to the accurate identification of the presence of ANCA, and analytic specificity refers to measurement of only the entity in question (ANCA), not confounded by the presence of other entities (antibodies).
Equally as important as analytic accuracy is the appropriate ordering of the tests in the right clinical setting. Using a test that is sensitive to the presence of a specific ANCA type accurately identifies the presence of either proteinase-3 (PR3)- or myeloperoxidase (MPO)-ANCA. Once obtained, test results must be evaluated in terms of their relationship to the diagnosis being considered. If the tests are deemed diagnostically useful based on the results, the data can be used to assess the positive and negative predictive value of the tests.
Immunofluorescence or antigen-specific testing—or both?
A definitive diagnosis is more likely if an immunofluorescence staining pattern of cANCA is paired with the antigen specificity of PR3-ANCA, for example, or a perinuclear immunofluorescence pattern (pANCA) is paired with a positive MPO-ANCA. When positive test pairings have been obtained and the patient’s antigen ANCA reactivity is known, subsequent serial ANCA testing with an antigen-specific assay alone may be indicated, because the ANCA types of patients with vasculitis are unlikely to switch between PR3 and MPO during the course of their disease. If matching pairings are not obtained, the diagnostic utility of the tests remains unconfirmed.
Antigen type (PR3 or MPO) is determined through antigen-specific methods that include solid-phase assays and other methods of bringing the specific antigen in contact with the specific antibody in question. There are two categories of solid-phase assays: the enzyme-linked immunoabsorbent assay (ELISA) and the capture ELISA. In the ELISA methodology, the antigen is directly coated to a plastic plate; in the capture ELISA, an anchor, usually a monoclonal antibody or combination of antibodies, captures the target antigen on the plate. In both ELISA and capture ELISA assays, ANCA contained in the serum sample subjected to testing bind to the immobilized antigen. The amount of ANCA bound to the antigen can then be detected by a secondary antibody that is conjugated with an enzyme that can elicit a color reaction. The intensity of the color reaction is proportional to the amount of ANCA bound to the immobilized antigen.
The ELISA methodology tends to trade off analytic sensitivity for specificity, since the antigen purification process (which allows the ELISA system to increase its specificity) can cause conformational changes to the antigen being bound to the plate. This, in turn, causes a loss of some recognition of the conformationally sensitive ANCA.
In capture ELISA, a specific antibody captures the antigen; this stabilizes the conformation, boosts the analytic sensitivity, and allows a gentler purification process because it only captures the antigen in question and then binds it to the plate. This process decreases false-positive test results caused by residual contaminants in the antigen preparation. Analytic sensitivity issues may come into play if the anchoring monoclonal antibody competes for the epitope on the antigen being recognized by the serum antibody in question (ANCA), causing occasional false-negative results.
Another method now applied to commercial ANCA testing involves bead-based multiplex assays. These assays are based on principles similar to the ELISA or capture ELISA methods. In multiplex microsphere technology, the purified antigen is bound to a polystyrene microsphere instead of a plate. The microsphere is then presented to the antibody in question. The bead is then introduced to a secondary antibody labeled with a fluorescent marker (instead of an enzyme) for detection of the antibody. One advantage of this system is that various beads containing different antigens can be introduced to the same serum sample, and then different color reactions can be measured for each bead. Because only one antigen is bound to each microsphere (eg, PR3-ANCA, MPOANCA or other specific autoantibodies), only specific antibodies will react to each bead of a specific color. If there is no MPO antibody in the sample, there will be no reaction against the MPO antigen bead; however, if PR3-ANCA is present in the sample, it would react with the PR3 antigen beads. Using this methodology, a single serum sample can be tested for a multitude of autoantibodies at the same time (see “Interpreting ANCA results: Accurate tests, appropriate orders,”2–10 above).

WHAT IS THE PROGNOSTIC VALUE OF SERIAL ANCA TESTING?
Persistent changes in ANCA levels in relapsing disease may have some value in predicting outcome. The issues to consider include the methodology used to determine serial ANCA levels, correlations between ANCA and disease activity, and the use of ANCA changes to guide treatment.
Does methodology matter when determining serial ANCA levels?
Methodology in serial ANCA testing is probably unimportant as long as the same method is used serially. Analysis of large groups of ANCA-positive patients show a statistically highly significant correlation among results obtained with different detection methods, including immunofluorescence, direct ELISA, or capture ELISA. However, at the individual patient level there is some variability.
Do ANCA levels correlate with disease activity?
In a prospective study, serial ANCA samples obtained during the Wegener’s Granulomatosis Etanercept Trial (WGET)11 were processed in the same manner (collected every 3 months, mean follow-up of 22 months, uniform handling of samples). All samples were analyzed by capture ELISA, and disease activity was measured by the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG). The results indicated that an increase in PR3-ANCA levels was not a significant predictor of relapse. The frequency of a relapse within 1 year of an increase in PR3-ANCA levels was found to be approximately 50%,11 a result similar to that reported in several smaller studies of different design and methodology.
Should ANCA changes guide treatment?
The available data regarding serial ANCA testing are limited mostly to PR3-ANCA. Serial ANCA testing has limited value as a guide to treatment and, in general, changes in ANCA levels alone should not be used to guide treatment decisions. In new patients without documented serial ANCA level associations, an increase in PR3-ANCA levels has no reliable predictive value. The existing literature suggests that this lack of association is not dependent on the method used for ANCA detection. For individual patients in whom long-term serial ANCA testing has been performed and a relationship between PR3-ANCA levels and disease activity has been established, serial ANCA testing can have some predictive value and can be used to guide treatment. For example, when remission is achieved by depleting B cells in patients with chronically relapsing GPA, ANCA levels usually go down. After B-cell reconstitution, the ANCA level rises in most patients, and this rise is usually associated with a flare shortly thereafter. A flare can be preempted when this pattern is determined in a specific patient, and preemptive treatment is applied accordingly.12
WHAT IS THE IMPLICATION OF ANCA TYPE?
Available reports consistently suggest that PR3-ANCA is associated with a higher mortality than MPO-ANCA (relative risk [RR], 3.78),13 and a higher relapse rate.14,15 A more rapid loss of renal function among patients with glomerulonephritis and PR3-ANCA than those with MPO-ANCA has also been reported.16 Using remission as the starting point, the number of days from complete remission to first disease flare was plotted for patients with MPO- versus PR3-ANCA in an analysis of long-term data from the Rituximab in ANCA-Associated Vasculitis (RAVE) trial.17 The resulting curve demonstrated a divergence in the probability of remaining in remission, confirming that remission maintenance is clearly greater in patients with MPO-ANCA than in patients with PR3-ANCA.
The primary end point of the RAVE trial was remission of disease without the use of prednisone at 6 months. There was little difference in end point achieved based on comparison of diagnosis (microscopic polyangiitis or granulomatosis) or treatment arms (rituximab versus cyclophosphamide); however, an analysis of end point data separating the patients by ANCA type showed that the treatment response to rituximab was superior to that of cyclophosphamide among patients with PR3-ANCA, whereas in patients with MPO-ANCA, there was little difference in response associated with either treatment. Regarding the likelihood of attaining an ANCA-negative status after 6 months, again MPO-ANCA patients showed no difference in frequency on either treatment. Among PR3-ANCA–positive patients, 50% in the rituximab arm attained ANCA-negative status compared with only 17% in the cyclophosphamide arm.17
SUMMARY
Diagnostic utility of ANCA testing depends on the methodology and clinical setting. Only cANCA/PR3-ANCA and pANCA/MPO-ANCA pairings have positive predictive value for diagnosis of small-vessel vasculitis. Mismatches in results, findings of human neutrophil elastase–ANCA, or identification of multiple positive antigens should be considered in cases of cocaine or drug use.
The clinical utility of serial ANCA testing is unconfirmed. Good data currently exist only for PR3-ANCA, and different drugs may affect ANCA levels in different ways. ANCA type is significant in that PR3-ANCA portends a higher relapse rate and poorer patient outcomes compared with MPO-ANCA.
- Russell KA, Wiegert E, Schroeder DR, Homburger HA, Specks U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin Immunol 2002; 103:196–203.
- Langford CA. The diagnostic utility of c-ANCA in Wegener’s granulomatosis. Cleve Clin J Med 1998; 65:135–140.
- Trimarchi M, Gregorini G, Facchetti F, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine 2001; 80:391–404.
- Wiesner O, Russell KA, Lee AS, et al. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum 2004; 50:2954–2965.
- Peikert T, Finkielman JD, Hummel AM, et al. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum 2008; 58:1546–1551.
- Knowles L, Buxton JA, Skuridina N, et al. Levamisole tainted cocaine causing severe neutropenia in Alberta and British Columbia. Harm Reduct J 2009; 6 (Nov 17):30. doi: 10.1186/1477-7517-6-30.
- Zhu NY, LeGatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med 2009; 150:287–289.
- Bradford M, Rosenberg B, Moreno J, Dumyati G. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med 2010; 152:758–759.
- Waller JM, Feramisco JD, Alberta-Wszolek L, McCalmont TH, Fox LP. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol 2010; 63:530–535. doi: 10.1016/j.jaad.2010.01.055
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant [published online ahead of print July 28, 2010]. Clin Pharmacol Ther 2010; 88:408–411. doi: 10.1038/clpt.2010.156
- Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med 2007; 147:611–619.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
- Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ; the Glomerular Disease Collaborative Network. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7:23–32.
- Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Franssen CFM, Gans ROB, Arends B, et al. Differences between anti-myeloperoxidase- and anti-proteinase 3-associated renal disease. Kidney Int 1995; 47:193–199.
- Stone JH, Merkel PA, Spiera R, et al; for the RAVE–ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
- Russell KA, Wiegert E, Schroeder DR, Homburger HA, Specks U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin Immunol 2002; 103:196–203.
- Langford CA. The diagnostic utility of c-ANCA in Wegener’s granulomatosis. Cleve Clin J Med 1998; 65:135–140.
- Trimarchi M, Gregorini G, Facchetti F, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine 2001; 80:391–404.
- Wiesner O, Russell KA, Lee AS, et al. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum 2004; 50:2954–2965.
- Peikert T, Finkielman JD, Hummel AM, et al. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum 2008; 58:1546–1551.
- Knowles L, Buxton JA, Skuridina N, et al. Levamisole tainted cocaine causing severe neutropenia in Alberta and British Columbia. Harm Reduct J 2009; 6 (Nov 17):30. doi: 10.1186/1477-7517-6-30.
- Zhu NY, LeGatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med 2009; 150:287–289.
- Bradford M, Rosenberg B, Moreno J, Dumyati G. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med 2010; 152:758–759.
- Waller JM, Feramisco JD, Alberta-Wszolek L, McCalmont TH, Fox LP. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol 2010; 63:530–535. doi: 10.1016/j.jaad.2010.01.055
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant [published online ahead of print July 28, 2010]. Clin Pharmacol Ther 2010; 88:408–411. doi: 10.1038/clpt.2010.156
- Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med 2007; 147:611–619.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
- Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ; the Glomerular Disease Collaborative Network. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7:23–32.
- Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Franssen CFM, Gans ROB, Arends B, et al. Differences between anti-myeloperoxidase- and anti-proteinase 3-associated renal disease. Kidney Int 1995; 47:193–199.
- Stone JH, Merkel PA, Spiera R, et al; for the RAVE–ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.