User login
- Cone biopsy typically includes the removal of the entire squamocolumnar junction of the cervix, generally agreed to be the site of origin of squamous cell carcinoma.
- Inject a premixed solution of 2% xylocaine and epinephrine in a concentration of 1:200,000 into the cervical stroma at 12 o’clock outside the intended margin.
- For a “cold-knife” cone, use a #11 surgical blade to begin a circular incision starting at 12 o’clock on the face of the cervix.
Cone biopsy of the cervix has been used for more than a century to rule out the presence of invasive carcinoma in women with squamous intraepithelial lesions (SIL). And while less invasive techniques such as colposcopy and loop electrosurgical excision procedures (LEEP) have reduced the need for diagnostic conization dramatically, cervical cone biopsy becomes necessary when these techniques prove inadequate.
Indications
When routine screening reveals abnormal cervical cytology such as atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesions (LGSIL), and high-grade squamous intraepithelial lesions (HGSIL), colposcopy and directed biopsy often are indicated. They help the physician rule out the presence of invasive carcinoma and determine the grade and distribution of the intraepithelial lesion. Currently, only HGSIL is considered premalignant and requires aggressive treatment via cone biopsy.1 Additional indications for the procedure are listed in Table 1.
Remove a single specimen that includes the entire transformation zone.
From a diagnostic standpoint, cone biopsy should be performed when the endocervical curettage is positive for dysplasia because it is difficult to grade the severity of dysplasia on the basis of the scant tissue fragments obtained by curettage. From a therapeutic standpoint, lesions that involve the endocervical canal are less likely to be adequately treated by destructive techniques such as cryotherapy. Thus, for most women, cone biopsy of the cervix is both diagnostic and therapeutic.
TABLE 1
Indications for cervical conization
|
Preparing for cone biopsy
Cone biopsy involves the surgical excision of a wedge-shaped portion of the ecto- and endocervix, including the removal of the entire squamocolumnar junction (SC junction) of the cervix, generally agreed to be the site of origin of squamous cell carcinoma of the cervix. The procedure may be performed using a scalpel, electroexcision (LEEP or fine-needle electrode), or CO2 laser. The choice between performing “cold-knife” versus “hot-knife” conization is largely one of personal preference and depends on surgical experience, the size/severity of the lesion, and the desires of the patient. In most cases, I prefer to use electrocautery because of its technical simplicity and the ability to operate with only a local anesthetic. It is particularly appropriate for patients with an obvious ectocervical lesion and in young, nulliparous women in whom I am trying to minimize the amount of healthy cervical tissue removed.
The use of colposcopy, either preoperatively or intraoperatively, allows precise evaluation of the amount of cervical tissue needed to be removed and reduces the incidence of positive margins. The geometry, i.e., width and depth, of the cone specimen will vary from patient to patient, depending on the size and location of the dysplastic lesion, as well as the location of the SC junction. Specifically, the width of the cone (ectocervical portion) is determined by the size of the transformation zone and size and location of any ectocervical lesions. The depth of the cone (endocervical portion) is determined by the location of the SC junction, the presence or absence of endocervi-cal disease, or the suspicion of a glandular lesion. When a discrete intraepithelial lesion has not been identified, it is critical to rule out a significant endocervical lesion. Therefore, the amount of tissue I plan to remove is based on the following 2 factors:
- Location of the SC junction. The more endocervical the SC junction is, the more likely the presence of a lesion higher in the canal. However, squamous intraepithelial lesions rarely extend higher than 2 cm into the canal.2
- Endocervical gland involvement. SIL frequently involves the endocervical glands, often to a depth of 5 mm or less. Several investigators have suggested that excision of endocervical glands to a depth of 7 to 10 mm produces acceptable cure rates.3
Based on these 2 principles, the endocer-vical portion of the cone should be 20 mm wide (10 mm on either side of the canal) and no more than 2 cm deep.
Technique
Traditionally, the first step in performing a cone biopsy is to place sutures lateral to the cervix to decrease bleeding (Figure 1). While many surgeons believe that these sutures have a beneficial effect on hemosta-sis, others have not found them to be necessary.4 And although cone biopsy typically is performed under regional or general anesthesia, I have found that IV sedation and injection of a local anesthetic are adequate for most cone biopsies.
Begin the procedure by placing either a posterior weighted speculum (for a cold-knife cone) or an insulated speculum (for electrocautery) into the vagina. Then inject a premixed solution of 2% xylocaine and epi-nephrine in a concentration of 1:200,000 into the cervical stroma at 12 o’clock outside the intended margin of the cone biopsy. Then grasp this tissue with a single-tooth tenacu-lum. Inject 5 to 10 cc of the solution para-cervically at 3 o’clock and again at 9 o’clock. I typically use a total of 20 to 30 cc to maintain hemostasis. Alternatively, the anesthetic can be infiltrated into the subepithelial stroma circumferentially around the ectocervix.
To perform a cold-knife cone, use a #11 surgical blade to begin a circular incision starting at 12 o’clock on the face of the cervix, angling the tip of the blade toward the endocervical canal (Figure 2). Use a uterine sound to mark a depth of 2 cm within the endo-cervical canal, typically the most cephalad margin of the cone. The goal is to remove a single specimen that includes the entire transformation zone and distal endocervical canal (Figure 3). After removing the cone, perform an endocervical curettage to collect an additional specimen. Apply light cautery to the edges of the cervical bed, as well as any bleeding points (Figure 4). Place 1 interrupted suture (I prefer 2-0 polyglactin) in each quadrant of the face of the cervix, starting from outside the margin of the cone and exiting near the cervical os. Then insert the suture near the cervical os and exit outside the margin of the cone. Tying these sut ures everts the cervical os to a degree and keeps the transformation zone closer to the external surface of the cervix, making it easier to find when performing future colposcopies.
Electroexcision with a fine-needle electrode is performed with a blend-1 waveform using 18 watts of power.5 Perform the excision using the “cut” button on the bovie handpiece and then use the leading edge of the electrical arc to incise the tissue. (It is best to use the arc, i.e., visible spark, to do the cutting rather than the tip of the instrument, which may “stick” to surrounding tissues.) Make a full circle to a depth of about 7 mm to outline the ectocervical cone margins. Then “pull down” the stroma on the edge of the specimen with a skin hook to continue the dissection parallel to the long axis of the endocervical canal to a depth of 1.5 to 2.0 cm. I excise the “base” of the cone with scissors to minimize any cautery effect at the endocervical margin. Achieve hemostasis by electrofulgurating the bleeding vessels using 30 to 40 watts of power in the coagulation mode. Monsel solution or paste then can be applied as needed. This technique is similar to CO2 laser conization, only it is easier to learn and quicker to perform.
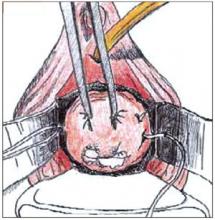
FIGURE 1
Begin the cone biopsy by placing lateral sutures at the cervicovaginal junction to decrease bleeding.
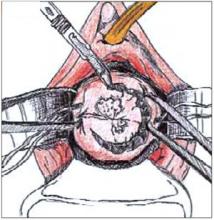
FIGURE 2
Use a #11 surgical blade to make the circular incision, angling the tip of the blade toward the endocervical canal.
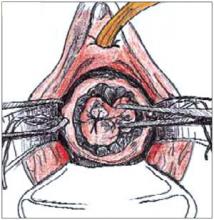
FIGURE 3
Grasp the specimen, including the entire transformation zone and distal endocervical canal, with an Allis clamp.
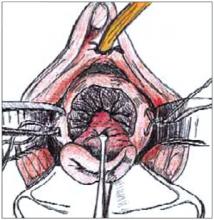
FIGURE 4
Complete the cone excision by cutting across endocervix. Apply light cautery to the edges of the cervical bed.
Follow-up
Postoperatively, patients should undergo cytologic screening every 4 months for 2 years to identify persistent or recurrent disease. Patients with positive cone biopsy margins face the highest risk of persistent or recurrent cervical intraepithelial neoplasia (CIN). Although there is considerable variation, studies generally have reported a 30% incidence of positive margins. The incidence of recurrent dysplasia when the margins are positive has been reported to be as high as 50%.6,7
In women with positive margins, colposcopy and cervical and endocervical cytology are an important part of the follow-up. I perform an endocervical curettage, in addition to a Pap smear, at each interval visit during the first year postoperatively and perform colposcopy at the 6- and 12- month visits. After 2 years of follow-up with normal results, cytologic screening should be performed annually, per routine care.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to the papillomavirus. N Engl J Med. 1992;327:1272-1278.
2. Burke L. Evolution of therapeutic approaches to cervical intraepithelial neoplasia. J Lower Genital Tract Disease. 1997;1:267-273.
3. Burke L, Covell L, Antonioli D. Carbon dioxide laser therapy of cervical intraepithelial neoplasia: factors determining success rates. Lasers Surg Med. 1980;1:113-122.
4. Gilbert L, Saunders NJ, Stringer R, Sharp F. Haemostasis and cold knife biopsy: a prospective randomized trial comparing a suture versus nonsuture technique. Obstet Gynecol. 1989;74:640-643.
5. Ferenczy A. Electroconization of the cervix with a fine-needle electrode. Obstet Gynecol. 1994;84:152-159.
6. AbdulKarim F, Nunez C. Cervical intraepithelial neoplasia after conization:a study of 522 consecutive cervical cones. Obstet Gynecol. 1985;65:77-81.
7. Lubicz S, Ezekwche C, Allen A, Schiffer M. Significance of cone biopsy margins in the management of patients with cervical neoplasia. J Reprod Med. 1984;29:178-184.
- Cone biopsy typically includes the removal of the entire squamocolumnar junction of the cervix, generally agreed to be the site of origin of squamous cell carcinoma.
- Inject a premixed solution of 2% xylocaine and epinephrine in a concentration of 1:200,000 into the cervical stroma at 12 o’clock outside the intended margin.
- For a “cold-knife” cone, use a #11 surgical blade to begin a circular incision starting at 12 o’clock on the face of the cervix.
Cone biopsy of the cervix has been used for more than a century to rule out the presence of invasive carcinoma in women with squamous intraepithelial lesions (SIL). And while less invasive techniques such as colposcopy and loop electrosurgical excision procedures (LEEP) have reduced the need for diagnostic conization dramatically, cervical cone biopsy becomes necessary when these techniques prove inadequate.
Indications
When routine screening reveals abnormal cervical cytology such as atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesions (LGSIL), and high-grade squamous intraepithelial lesions (HGSIL), colposcopy and directed biopsy often are indicated. They help the physician rule out the presence of invasive carcinoma and determine the grade and distribution of the intraepithelial lesion. Currently, only HGSIL is considered premalignant and requires aggressive treatment via cone biopsy.1 Additional indications for the procedure are listed in Table 1.
Remove a single specimen that includes the entire transformation zone.
From a diagnostic standpoint, cone biopsy should be performed when the endocervical curettage is positive for dysplasia because it is difficult to grade the severity of dysplasia on the basis of the scant tissue fragments obtained by curettage. From a therapeutic standpoint, lesions that involve the endocervical canal are less likely to be adequately treated by destructive techniques such as cryotherapy. Thus, for most women, cone biopsy of the cervix is both diagnostic and therapeutic.
TABLE 1
Indications for cervical conization
|
Preparing for cone biopsy
Cone biopsy involves the surgical excision of a wedge-shaped portion of the ecto- and endocervix, including the removal of the entire squamocolumnar junction (SC junction) of the cervix, generally agreed to be the site of origin of squamous cell carcinoma of the cervix. The procedure may be performed using a scalpel, electroexcision (LEEP or fine-needle electrode), or CO2 laser. The choice between performing “cold-knife” versus “hot-knife” conization is largely one of personal preference and depends on surgical experience, the size/severity of the lesion, and the desires of the patient. In most cases, I prefer to use electrocautery because of its technical simplicity and the ability to operate with only a local anesthetic. It is particularly appropriate for patients with an obvious ectocervical lesion and in young, nulliparous women in whom I am trying to minimize the amount of healthy cervical tissue removed.
The use of colposcopy, either preoperatively or intraoperatively, allows precise evaluation of the amount of cervical tissue needed to be removed and reduces the incidence of positive margins. The geometry, i.e., width and depth, of the cone specimen will vary from patient to patient, depending on the size and location of the dysplastic lesion, as well as the location of the SC junction. Specifically, the width of the cone (ectocervical portion) is determined by the size of the transformation zone and size and location of any ectocervical lesions. The depth of the cone (endocervical portion) is determined by the location of the SC junction, the presence or absence of endocervi-cal disease, or the suspicion of a glandular lesion. When a discrete intraepithelial lesion has not been identified, it is critical to rule out a significant endocervical lesion. Therefore, the amount of tissue I plan to remove is based on the following 2 factors:
- Location of the SC junction. The more endocervical the SC junction is, the more likely the presence of a lesion higher in the canal. However, squamous intraepithelial lesions rarely extend higher than 2 cm into the canal.2
- Endocervical gland involvement. SIL frequently involves the endocervical glands, often to a depth of 5 mm or less. Several investigators have suggested that excision of endocervical glands to a depth of 7 to 10 mm produces acceptable cure rates.3
Based on these 2 principles, the endocer-vical portion of the cone should be 20 mm wide (10 mm on either side of the canal) and no more than 2 cm deep.
Technique
Traditionally, the first step in performing a cone biopsy is to place sutures lateral to the cervix to decrease bleeding (Figure 1). While many surgeons believe that these sutures have a beneficial effect on hemosta-sis, others have not found them to be necessary.4 And although cone biopsy typically is performed under regional or general anesthesia, I have found that IV sedation and injection of a local anesthetic are adequate for most cone biopsies.
Begin the procedure by placing either a posterior weighted speculum (for a cold-knife cone) or an insulated speculum (for electrocautery) into the vagina. Then inject a premixed solution of 2% xylocaine and epi-nephrine in a concentration of 1:200,000 into the cervical stroma at 12 o’clock outside the intended margin of the cone biopsy. Then grasp this tissue with a single-tooth tenacu-lum. Inject 5 to 10 cc of the solution para-cervically at 3 o’clock and again at 9 o’clock. I typically use a total of 20 to 30 cc to maintain hemostasis. Alternatively, the anesthetic can be infiltrated into the subepithelial stroma circumferentially around the ectocervix.
To perform a cold-knife cone, use a #11 surgical blade to begin a circular incision starting at 12 o’clock on the face of the cervix, angling the tip of the blade toward the endocervical canal (Figure 2). Use a uterine sound to mark a depth of 2 cm within the endo-cervical canal, typically the most cephalad margin of the cone. The goal is to remove a single specimen that includes the entire transformation zone and distal endocervical canal (Figure 3). After removing the cone, perform an endocervical curettage to collect an additional specimen. Apply light cautery to the edges of the cervical bed, as well as any bleeding points (Figure 4). Place 1 interrupted suture (I prefer 2-0 polyglactin) in each quadrant of the face of the cervix, starting from outside the margin of the cone and exiting near the cervical os. Then insert the suture near the cervical os and exit outside the margin of the cone. Tying these sut ures everts the cervical os to a degree and keeps the transformation zone closer to the external surface of the cervix, making it easier to find when performing future colposcopies.
Electroexcision with a fine-needle electrode is performed with a blend-1 waveform using 18 watts of power.5 Perform the excision using the “cut” button on the bovie handpiece and then use the leading edge of the electrical arc to incise the tissue. (It is best to use the arc, i.e., visible spark, to do the cutting rather than the tip of the instrument, which may “stick” to surrounding tissues.) Make a full circle to a depth of about 7 mm to outline the ectocervical cone margins. Then “pull down” the stroma on the edge of the specimen with a skin hook to continue the dissection parallel to the long axis of the endocervical canal to a depth of 1.5 to 2.0 cm. I excise the “base” of the cone with scissors to minimize any cautery effect at the endocervical margin. Achieve hemostasis by electrofulgurating the bleeding vessels using 30 to 40 watts of power in the coagulation mode. Monsel solution or paste then can be applied as needed. This technique is similar to CO2 laser conization, only it is easier to learn and quicker to perform.
FIGURE 1
Begin the cone biopsy by placing lateral sutures at the cervicovaginal junction to decrease bleeding.
FIGURE 2
Use a #11 surgical blade to make the circular incision, angling the tip of the blade toward the endocervical canal.
FIGURE 3
Grasp the specimen, including the entire transformation zone and distal endocervical canal, with an Allis clamp.
FIGURE 4
Complete the cone excision by cutting across endocervix. Apply light cautery to the edges of the cervical bed.
Follow-up
Postoperatively, patients should undergo cytologic screening every 4 months for 2 years to identify persistent or recurrent disease. Patients with positive cone biopsy margins face the highest risk of persistent or recurrent cervical intraepithelial neoplasia (CIN). Although there is considerable variation, studies generally have reported a 30% incidence of positive margins. The incidence of recurrent dysplasia when the margins are positive has been reported to be as high as 50%.6,7
In women with positive margins, colposcopy and cervical and endocervical cytology are an important part of the follow-up. I perform an endocervical curettage, in addition to a Pap smear, at each interval visit during the first year postoperatively and perform colposcopy at the 6- and 12- month visits. After 2 years of follow-up with normal results, cytologic screening should be performed annually, per routine care.
The author reports no financial relationship with any companies whose products are mentioned in this article.
- Cone biopsy typically includes the removal of the entire squamocolumnar junction of the cervix, generally agreed to be the site of origin of squamous cell carcinoma.
- Inject a premixed solution of 2% xylocaine and epinephrine in a concentration of 1:200,000 into the cervical stroma at 12 o’clock outside the intended margin.
- For a “cold-knife” cone, use a #11 surgical blade to begin a circular incision starting at 12 o’clock on the face of the cervix.
Cone biopsy of the cervix has been used for more than a century to rule out the presence of invasive carcinoma in women with squamous intraepithelial lesions (SIL). And while less invasive techniques such as colposcopy and loop electrosurgical excision procedures (LEEP) have reduced the need for diagnostic conization dramatically, cervical cone biopsy becomes necessary when these techniques prove inadequate.
Indications
When routine screening reveals abnormal cervical cytology such as atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesions (LGSIL), and high-grade squamous intraepithelial lesions (HGSIL), colposcopy and directed biopsy often are indicated. They help the physician rule out the presence of invasive carcinoma and determine the grade and distribution of the intraepithelial lesion. Currently, only HGSIL is considered premalignant and requires aggressive treatment via cone biopsy.1 Additional indications for the procedure are listed in Table 1.
Remove a single specimen that includes the entire transformation zone.
From a diagnostic standpoint, cone biopsy should be performed when the endocervical curettage is positive for dysplasia because it is difficult to grade the severity of dysplasia on the basis of the scant tissue fragments obtained by curettage. From a therapeutic standpoint, lesions that involve the endocervical canal are less likely to be adequately treated by destructive techniques such as cryotherapy. Thus, for most women, cone biopsy of the cervix is both diagnostic and therapeutic.
TABLE 1
Indications for cervical conization
|
Preparing for cone biopsy
Cone biopsy involves the surgical excision of a wedge-shaped portion of the ecto- and endocervix, including the removal of the entire squamocolumnar junction (SC junction) of the cervix, generally agreed to be the site of origin of squamous cell carcinoma of the cervix. The procedure may be performed using a scalpel, electroexcision (LEEP or fine-needle electrode), or CO2 laser. The choice between performing “cold-knife” versus “hot-knife” conization is largely one of personal preference and depends on surgical experience, the size/severity of the lesion, and the desires of the patient. In most cases, I prefer to use electrocautery because of its technical simplicity and the ability to operate with only a local anesthetic. It is particularly appropriate for patients with an obvious ectocervical lesion and in young, nulliparous women in whom I am trying to minimize the amount of healthy cervical tissue removed.
The use of colposcopy, either preoperatively or intraoperatively, allows precise evaluation of the amount of cervical tissue needed to be removed and reduces the incidence of positive margins. The geometry, i.e., width and depth, of the cone specimen will vary from patient to patient, depending on the size and location of the dysplastic lesion, as well as the location of the SC junction. Specifically, the width of the cone (ectocervical portion) is determined by the size of the transformation zone and size and location of any ectocervical lesions. The depth of the cone (endocervical portion) is determined by the location of the SC junction, the presence or absence of endocervi-cal disease, or the suspicion of a glandular lesion. When a discrete intraepithelial lesion has not been identified, it is critical to rule out a significant endocervical lesion. Therefore, the amount of tissue I plan to remove is based on the following 2 factors:
- Location of the SC junction. The more endocervical the SC junction is, the more likely the presence of a lesion higher in the canal. However, squamous intraepithelial lesions rarely extend higher than 2 cm into the canal.2
- Endocervical gland involvement. SIL frequently involves the endocervical glands, often to a depth of 5 mm or less. Several investigators have suggested that excision of endocervical glands to a depth of 7 to 10 mm produces acceptable cure rates.3
Based on these 2 principles, the endocer-vical portion of the cone should be 20 mm wide (10 mm on either side of the canal) and no more than 2 cm deep.
Technique
Traditionally, the first step in performing a cone biopsy is to place sutures lateral to the cervix to decrease bleeding (Figure 1). While many surgeons believe that these sutures have a beneficial effect on hemosta-sis, others have not found them to be necessary.4 And although cone biopsy typically is performed under regional or general anesthesia, I have found that IV sedation and injection of a local anesthetic are adequate for most cone biopsies.
Begin the procedure by placing either a posterior weighted speculum (for a cold-knife cone) or an insulated speculum (for electrocautery) into the vagina. Then inject a premixed solution of 2% xylocaine and epi-nephrine in a concentration of 1:200,000 into the cervical stroma at 12 o’clock outside the intended margin of the cone biopsy. Then grasp this tissue with a single-tooth tenacu-lum. Inject 5 to 10 cc of the solution para-cervically at 3 o’clock and again at 9 o’clock. I typically use a total of 20 to 30 cc to maintain hemostasis. Alternatively, the anesthetic can be infiltrated into the subepithelial stroma circumferentially around the ectocervix.
To perform a cold-knife cone, use a #11 surgical blade to begin a circular incision starting at 12 o’clock on the face of the cervix, angling the tip of the blade toward the endocervical canal (Figure 2). Use a uterine sound to mark a depth of 2 cm within the endo-cervical canal, typically the most cephalad margin of the cone. The goal is to remove a single specimen that includes the entire transformation zone and distal endocervical canal (Figure 3). After removing the cone, perform an endocervical curettage to collect an additional specimen. Apply light cautery to the edges of the cervical bed, as well as any bleeding points (Figure 4). Place 1 interrupted suture (I prefer 2-0 polyglactin) in each quadrant of the face of the cervix, starting from outside the margin of the cone and exiting near the cervical os. Then insert the suture near the cervical os and exit outside the margin of the cone. Tying these sut ures everts the cervical os to a degree and keeps the transformation zone closer to the external surface of the cervix, making it easier to find when performing future colposcopies.
Electroexcision with a fine-needle electrode is performed with a blend-1 waveform using 18 watts of power.5 Perform the excision using the “cut” button on the bovie handpiece and then use the leading edge of the electrical arc to incise the tissue. (It is best to use the arc, i.e., visible spark, to do the cutting rather than the tip of the instrument, which may “stick” to surrounding tissues.) Make a full circle to a depth of about 7 mm to outline the ectocervical cone margins. Then “pull down” the stroma on the edge of the specimen with a skin hook to continue the dissection parallel to the long axis of the endocervical canal to a depth of 1.5 to 2.0 cm. I excise the “base” of the cone with scissors to minimize any cautery effect at the endocervical margin. Achieve hemostasis by electrofulgurating the bleeding vessels using 30 to 40 watts of power in the coagulation mode. Monsel solution or paste then can be applied as needed. This technique is similar to CO2 laser conization, only it is easier to learn and quicker to perform.
FIGURE 1
Begin the cone biopsy by placing lateral sutures at the cervicovaginal junction to decrease bleeding.
FIGURE 2
Use a #11 surgical blade to make the circular incision, angling the tip of the blade toward the endocervical canal.
FIGURE 3
Grasp the specimen, including the entire transformation zone and distal endocervical canal, with an Allis clamp.
FIGURE 4
Complete the cone excision by cutting across endocervix. Apply light cautery to the edges of the cervical bed.
Follow-up
Postoperatively, patients should undergo cytologic screening every 4 months for 2 years to identify persistent or recurrent disease. Patients with positive cone biopsy margins face the highest risk of persistent or recurrent cervical intraepithelial neoplasia (CIN). Although there is considerable variation, studies generally have reported a 30% incidence of positive margins. The incidence of recurrent dysplasia when the margins are positive has been reported to be as high as 50%.6,7
In women with positive margins, colposcopy and cervical and endocervical cytology are an important part of the follow-up. I perform an endocervical curettage, in addition to a Pap smear, at each interval visit during the first year postoperatively and perform colposcopy at the 6- and 12- month visits. After 2 years of follow-up with normal results, cytologic screening should be performed annually, per routine care.
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to the papillomavirus. N Engl J Med. 1992;327:1272-1278.
2. Burke L. Evolution of therapeutic approaches to cervical intraepithelial neoplasia. J Lower Genital Tract Disease. 1997;1:267-273.
3. Burke L, Covell L, Antonioli D. Carbon dioxide laser therapy of cervical intraepithelial neoplasia: factors determining success rates. Lasers Surg Med. 1980;1:113-122.
4. Gilbert L, Saunders NJ, Stringer R, Sharp F. Haemostasis and cold knife biopsy: a prospective randomized trial comparing a suture versus nonsuture technique. Obstet Gynecol. 1989;74:640-643.
5. Ferenczy A. Electroconization of the cervix with a fine-needle electrode. Obstet Gynecol. 1994;84:152-159.
6. AbdulKarim F, Nunez C. Cervical intraepithelial neoplasia after conization:a study of 522 consecutive cervical cones. Obstet Gynecol. 1985;65:77-81.
7. Lubicz S, Ezekwche C, Allen A, Schiffer M. Significance of cone biopsy margins in the management of patients with cervical neoplasia. J Reprod Med. 1984;29:178-184.
1. Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to the papillomavirus. N Engl J Med. 1992;327:1272-1278.
2. Burke L. Evolution of therapeutic approaches to cervical intraepithelial neoplasia. J Lower Genital Tract Disease. 1997;1:267-273.
3. Burke L, Covell L, Antonioli D. Carbon dioxide laser therapy of cervical intraepithelial neoplasia: factors determining success rates. Lasers Surg Med. 1980;1:113-122.
4. Gilbert L, Saunders NJ, Stringer R, Sharp F. Haemostasis and cold knife biopsy: a prospective randomized trial comparing a suture versus nonsuture technique. Obstet Gynecol. 1989;74:640-643.
5. Ferenczy A. Electroconization of the cervix with a fine-needle electrode. Obstet Gynecol. 1994;84:152-159.
6. AbdulKarim F, Nunez C. Cervical intraepithelial neoplasia after conization:a study of 522 consecutive cervical cones. Obstet Gynecol. 1985;65:77-81.
7. Lubicz S, Ezekwche C, Allen A, Schiffer M. Significance of cone biopsy margins in the management of patients with cervical neoplasia. J Reprod Med. 1984;29:178-184.