User login
Colonic diaphragm disease (CDD) is a rare but serious complication of nonsteroidal anti‐inflammatory drug (NSAID) use in which diaphragm‐like strictures develop in the large and/or small intestines. There have been about 100 reported cases of CDD since this finding was first reported in the medical literature in 1989.1,2 However, given the frequency of NSAID use, providers should consider this diagnosis.
Case Report
An 85-year‐old woman presented to the emergency department (ED) with generalized weakness, occult positive stool, and severe anemia requiring blood transfusions. Eight months prior, she had presented to her primary care physician (PCP) for a routine visit and was found to have anemia. She was referred to gastroenterology by her PCP for an upper endoscopy but refused the referral despite clear warnings of the potential for serious complications. She was started on ferrous sulfate 325 mg po tid at that time. The patient’s hemoglobin (Hbg) levels ranged from 10.8 to 11.2 g/dL prior to her ED presentation, and random fecal occult blood testing had been negative.
In the ED, the patient reported no abdominal pain, nausea emesis or constipation. Her past medical history was significant for compensated diastolic heart failure, paroxysmal atrial fibrillation controlled by medication, and chronic lumbago. The patient had been prescribed NSAIDs for 10 years for the chronic lumbago diagnosis; however, she was inconsistent in taking this medication until more recent years. Surgical history included a hysterectomy and back surgeries in the distant past. She had no symptoms or history of inflammatory bowel disease. The patient’s medications included both the oral and topical forms of diclofenac, conjugated estrogen, nitroglycerin, amlodipine, hydrocodone bitartrate and acetaminophen 5 mg once daily, amiodarone, diazepam, valsartan, furosemide, oxycodone 5 mg nightly prn for severe pain, and ferrous sulfate 325 mg.
Significant laboratory results in the ED included a Hbg level of 8.3 g/dL; a ferritin level of 11.9 ng/mL, and a serum iron level of 12 mg/dL. A computerized tomography scan of the abdomen and pelvis was normal. The patient was admitted, received a blood transfusion, and a gastroenterology consult was obtained for an upper and lower endoscopy. Although copy was normal, the colonoscopy revealed evidence of CDD of the ascending colon.
Colonic Diaphragm Disease
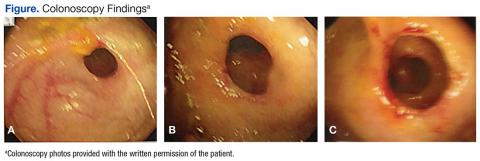
The gross findings seen on this patient’s colonoscopy showed evidence of the development of an internal colonic stricture from the fibrous overgrowth of the diaphragm (Figures A, B, & C). The narrow lumen is exhibited particularly well in Figure A in the upper left image. The external surface of the intestine appears normal.
Presentation and Evaluation
The presenting symptoms of CDD can vary and include abdominal pain, vomiting, lower gastrointestinal bleeding, anemia, and intestinal obstruction/perforation. Evidence seems to suggest a greater association with use of the oxicams (meloxicam, piroxicam) or diclofenac.1,2 Some researchers have suggested an association with long‐acting NSAID formulations.1 The use of misoprostol or proton pump inhibitors does not seem to lessen the chance of stricture formulation.2 Cox‐2 inhibitors are less likely players in the development of CDD.2 Most diaphragms in the large colon have been noted in the cecum, ascending and transverse colon, though descending colonic lesions have been documented as well.1
The incidence of CDD is higher among women and occurs most often in the seventh decade of life. Correlation with the duration of NSAID use prior to disease onset varies widely, with studies documenting disease onset from 3 months to 5 years after initiating consistent NSAID use.1 The patient in this case study was prescribed NSAIDs for almost 10 years, although she had poor adherence. One study also notes a possible association of CYP2C9*3 genetic polymorphism with the propensity to develop CDD. This finding is particularly interesting since NSAIDs are principally metabolized by CYP2C9 in the liver. CYP2C9 polymorphisms are thought to induce higher plasma concentrations of NSAIDs over time, which may lead to the formation of colonic diaphragms.2
Endoscopy with biopsy is the diagnostic modality of choice. Use of capsule endoscopy is limited because the capsule may not be able to pass through narrow stricture walls.
Pathology and Histology
The mechanism by which NSAIDs induce the formation of colonic diaphragms may be related to these medications’ effects on mucosal integrity, vasoconstriction, and vascular spasm. These effects may lead to “erosions, ulceration, and diaphragm-like strictures.”3
The primary histologic feature of these strictures is submucosal fibrosis with an intact muscularis propria. Prominent features also include areas of ulceration and granulation.1 The widened submucosa results in annular constriction of the intestinal lumen with disorganized bundles of smooth muscle, unmyelinated nerve bundles, scattered ganglion cells, and blood vessels. This morphology can resemble a node or mass.4 The mucosa is typically normal.5 Conditions such as vascular and neuromuscular hamartoma can resemble CDD. Interestingly, the fibrous changes in CDD have been noted apart from NSAID use, leading to speculation that diaphragm formation may be a result of injury and not exclusively a reaction to NSAID use.5
Treatment
Recommendations include NSAID withdrawal, endoscopic/fluoroscopic dilatation, and surgical resection. Outcomes with these treatment approaches have been highly variable. For some patients, NSAID withdrawal alone or following resection has resulted in success. However, even with withdrawal of NSAIDs, symptoms may improve, although the histologic findings of CDD may not be reversed.3,6 In one case report, a colonic stricture persisted endoscopically after NSAID withdrawal but resolved after a 20-week course of prednisone.7
Conclusion
Despite NSAID cessation, the patient in this case study presented a month later with an acute bowel obstruction. The bowel obstruction responded to conservative treatment, and the patient declined surgical intervention. The patient was readmitted again 4 months later with an acute bowel obstruction, and underwent an emergent colonic resection. The pathology report was consistent with CDD. In the 3 years since the resection, the patient has had no recurrences and continues to avoid all NSAIDs. She has refused a follow-up postresection colonoscopy.
1. Munipalle PC, Garud T, Light D. Diaphragmatic disease of the colon: systematic review. Colorectal Dis. 2013;15(9):1063-1069.
2. Ishihara M, Ohmiya N, Nakamura M, et al. Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther. 2014;40(5):538-547.
3. Keeling AN, Davis JL, Williams A, Sabharwal T, Adam A. Fluoroscopically guided balloon dilation of NSAID-induced colonic diaphragm. J Vasc Interv Radiol. 2007;18(8):1060-1062.
4. Yousfi MM, De Petris G, Leighton JA, et al. Diaphragm disease after use of nonsteroidal anti-inflammatory agents: first report of diagnosis with capsule endoscopy. J Clin Gastroenterol. 2004;38(8):686-691.
5. Pilgrim S, Velchuru V, Waters G, Tsiamis A, Lal R. Diaphragm disease and small bowel enteropathy due to nonsteroidal anti-inflammatory drugs: a surgical perspective. Colorectal Dis. 2011;13(4):463-466.
6. Munipalle PC, Little M, Garud T, Henderson D. NSAID-induced diaphragmatic disease of the colon. BMJ Case Rep. 2013;2013:pii:bcr2012008448.
7. Penner RM, Williams CN. Resolution of multiple severe nonsteroidal anti-inflammatory drug-induced colonic strictures with prednisone therapy: a case report and review of the literature. Can J Gastroenterol. 2003;17(8):497-500.
Colonic diaphragm disease (CDD) is a rare but serious complication of nonsteroidal anti‐inflammatory drug (NSAID) use in which diaphragm‐like strictures develop in the large and/or small intestines. There have been about 100 reported cases of CDD since this finding was first reported in the medical literature in 1989.1,2 However, given the frequency of NSAID use, providers should consider this diagnosis.
Case Report
An 85-year‐old woman presented to the emergency department (ED) with generalized weakness, occult positive stool, and severe anemia requiring blood transfusions. Eight months prior, she had presented to her primary care physician (PCP) for a routine visit and was found to have anemia. She was referred to gastroenterology by her PCP for an upper endoscopy but refused the referral despite clear warnings of the potential for serious complications. She was started on ferrous sulfate 325 mg po tid at that time. The patient’s hemoglobin (Hbg) levels ranged from 10.8 to 11.2 g/dL prior to her ED presentation, and random fecal occult blood testing had been negative.
In the ED, the patient reported no abdominal pain, nausea emesis or constipation. Her past medical history was significant for compensated diastolic heart failure, paroxysmal atrial fibrillation controlled by medication, and chronic lumbago. The patient had been prescribed NSAIDs for 10 years for the chronic lumbago diagnosis; however, she was inconsistent in taking this medication until more recent years. Surgical history included a hysterectomy and back surgeries in the distant past. She had no symptoms or history of inflammatory bowel disease. The patient’s medications included both the oral and topical forms of diclofenac, conjugated estrogen, nitroglycerin, amlodipine, hydrocodone bitartrate and acetaminophen 5 mg once daily, amiodarone, diazepam, valsartan, furosemide, oxycodone 5 mg nightly prn for severe pain, and ferrous sulfate 325 mg.
Significant laboratory results in the ED included a Hbg level of 8.3 g/dL; a ferritin level of 11.9 ng/mL, and a serum iron level of 12 mg/dL. A computerized tomography scan of the abdomen and pelvis was normal. The patient was admitted, received a blood transfusion, and a gastroenterology consult was obtained for an upper and lower endoscopy. Although copy was normal, the colonoscopy revealed evidence of CDD of the ascending colon.
Colonic Diaphragm Disease
The gross findings seen on this patient’s colonoscopy showed evidence of the development of an internal colonic stricture from the fibrous overgrowth of the diaphragm (Figures A, B, & C). The narrow lumen is exhibited particularly well in Figure A in the upper left image. The external surface of the intestine appears normal.
Presentation and Evaluation
The presenting symptoms of CDD can vary and include abdominal pain, vomiting, lower gastrointestinal bleeding, anemia, and intestinal obstruction/perforation. Evidence seems to suggest a greater association with use of the oxicams (meloxicam, piroxicam) or diclofenac.1,2 Some researchers have suggested an association with long‐acting NSAID formulations.1 The use of misoprostol or proton pump inhibitors does not seem to lessen the chance of stricture formulation.2 Cox‐2 inhibitors are less likely players in the development of CDD.2 Most diaphragms in the large colon have been noted in the cecum, ascending and transverse colon, though descending colonic lesions have been documented as well.1
The incidence of CDD is higher among women and occurs most often in the seventh decade of life. Correlation with the duration of NSAID use prior to disease onset varies widely, with studies documenting disease onset from 3 months to 5 years after initiating consistent NSAID use.1 The patient in this case study was prescribed NSAIDs for almost 10 years, although she had poor adherence. One study also notes a possible association of CYP2C9*3 genetic polymorphism with the propensity to develop CDD. This finding is particularly interesting since NSAIDs are principally metabolized by CYP2C9 in the liver. CYP2C9 polymorphisms are thought to induce higher plasma concentrations of NSAIDs over time, which may lead to the formation of colonic diaphragms.2
Endoscopy with biopsy is the diagnostic modality of choice. Use of capsule endoscopy is limited because the capsule may not be able to pass through narrow stricture walls.
Pathology and Histology
The mechanism by which NSAIDs induce the formation of colonic diaphragms may be related to these medications’ effects on mucosal integrity, vasoconstriction, and vascular spasm. These effects may lead to “erosions, ulceration, and diaphragm-like strictures.”3
The primary histologic feature of these strictures is submucosal fibrosis with an intact muscularis propria. Prominent features also include areas of ulceration and granulation.1 The widened submucosa results in annular constriction of the intestinal lumen with disorganized bundles of smooth muscle, unmyelinated nerve bundles, scattered ganglion cells, and blood vessels. This morphology can resemble a node or mass.4 The mucosa is typically normal.5 Conditions such as vascular and neuromuscular hamartoma can resemble CDD. Interestingly, the fibrous changes in CDD have been noted apart from NSAID use, leading to speculation that diaphragm formation may be a result of injury and not exclusively a reaction to NSAID use.5
Treatment
Recommendations include NSAID withdrawal, endoscopic/fluoroscopic dilatation, and surgical resection. Outcomes with these treatment approaches have been highly variable. For some patients, NSAID withdrawal alone or following resection has resulted in success. However, even with withdrawal of NSAIDs, symptoms may improve, although the histologic findings of CDD may not be reversed.3,6 In one case report, a colonic stricture persisted endoscopically after NSAID withdrawal but resolved after a 20-week course of prednisone.7
Conclusion
Despite NSAID cessation, the patient in this case study presented a month later with an acute bowel obstruction. The bowel obstruction responded to conservative treatment, and the patient declined surgical intervention. The patient was readmitted again 4 months later with an acute bowel obstruction, and underwent an emergent colonic resection. The pathology report was consistent with CDD. In the 3 years since the resection, the patient has had no recurrences and continues to avoid all NSAIDs. She has refused a follow-up postresection colonoscopy.
Colonic diaphragm disease (CDD) is a rare but serious complication of nonsteroidal anti‐inflammatory drug (NSAID) use in which diaphragm‐like strictures develop in the large and/or small intestines. There have been about 100 reported cases of CDD since this finding was first reported in the medical literature in 1989.1,2 However, given the frequency of NSAID use, providers should consider this diagnosis.
Case Report
An 85-year‐old woman presented to the emergency department (ED) with generalized weakness, occult positive stool, and severe anemia requiring blood transfusions. Eight months prior, she had presented to her primary care physician (PCP) for a routine visit and was found to have anemia. She was referred to gastroenterology by her PCP for an upper endoscopy but refused the referral despite clear warnings of the potential for serious complications. She was started on ferrous sulfate 325 mg po tid at that time. The patient’s hemoglobin (Hbg) levels ranged from 10.8 to 11.2 g/dL prior to her ED presentation, and random fecal occult blood testing had been negative.
In the ED, the patient reported no abdominal pain, nausea emesis or constipation. Her past medical history was significant for compensated diastolic heart failure, paroxysmal atrial fibrillation controlled by medication, and chronic lumbago. The patient had been prescribed NSAIDs for 10 years for the chronic lumbago diagnosis; however, she was inconsistent in taking this medication until more recent years. Surgical history included a hysterectomy and back surgeries in the distant past. She had no symptoms or history of inflammatory bowel disease. The patient’s medications included both the oral and topical forms of diclofenac, conjugated estrogen, nitroglycerin, amlodipine, hydrocodone bitartrate and acetaminophen 5 mg once daily, amiodarone, diazepam, valsartan, furosemide, oxycodone 5 mg nightly prn for severe pain, and ferrous sulfate 325 mg.
Significant laboratory results in the ED included a Hbg level of 8.3 g/dL; a ferritin level of 11.9 ng/mL, and a serum iron level of 12 mg/dL. A computerized tomography scan of the abdomen and pelvis was normal. The patient was admitted, received a blood transfusion, and a gastroenterology consult was obtained for an upper and lower endoscopy. Although copy was normal, the colonoscopy revealed evidence of CDD of the ascending colon.
Colonic Diaphragm Disease
The gross findings seen on this patient’s colonoscopy showed evidence of the development of an internal colonic stricture from the fibrous overgrowth of the diaphragm (Figures A, B, & C). The narrow lumen is exhibited particularly well in Figure A in the upper left image. The external surface of the intestine appears normal.
Presentation and Evaluation
The presenting symptoms of CDD can vary and include abdominal pain, vomiting, lower gastrointestinal bleeding, anemia, and intestinal obstruction/perforation. Evidence seems to suggest a greater association with use of the oxicams (meloxicam, piroxicam) or diclofenac.1,2 Some researchers have suggested an association with long‐acting NSAID formulations.1 The use of misoprostol or proton pump inhibitors does not seem to lessen the chance of stricture formulation.2 Cox‐2 inhibitors are less likely players in the development of CDD.2 Most diaphragms in the large colon have been noted in the cecum, ascending and transverse colon, though descending colonic lesions have been documented as well.1
The incidence of CDD is higher among women and occurs most often in the seventh decade of life. Correlation with the duration of NSAID use prior to disease onset varies widely, with studies documenting disease onset from 3 months to 5 years after initiating consistent NSAID use.1 The patient in this case study was prescribed NSAIDs for almost 10 years, although she had poor adherence. One study also notes a possible association of CYP2C9*3 genetic polymorphism with the propensity to develop CDD. This finding is particularly interesting since NSAIDs are principally metabolized by CYP2C9 in the liver. CYP2C9 polymorphisms are thought to induce higher plasma concentrations of NSAIDs over time, which may lead to the formation of colonic diaphragms.2
Endoscopy with biopsy is the diagnostic modality of choice. Use of capsule endoscopy is limited because the capsule may not be able to pass through narrow stricture walls.
Pathology and Histology
The mechanism by which NSAIDs induce the formation of colonic diaphragms may be related to these medications’ effects on mucosal integrity, vasoconstriction, and vascular spasm. These effects may lead to “erosions, ulceration, and diaphragm-like strictures.”3
The primary histologic feature of these strictures is submucosal fibrosis with an intact muscularis propria. Prominent features also include areas of ulceration and granulation.1 The widened submucosa results in annular constriction of the intestinal lumen with disorganized bundles of smooth muscle, unmyelinated nerve bundles, scattered ganglion cells, and blood vessels. This morphology can resemble a node or mass.4 The mucosa is typically normal.5 Conditions such as vascular and neuromuscular hamartoma can resemble CDD. Interestingly, the fibrous changes in CDD have been noted apart from NSAID use, leading to speculation that diaphragm formation may be a result of injury and not exclusively a reaction to NSAID use.5
Treatment
Recommendations include NSAID withdrawal, endoscopic/fluoroscopic dilatation, and surgical resection. Outcomes with these treatment approaches have been highly variable. For some patients, NSAID withdrawal alone or following resection has resulted in success. However, even with withdrawal of NSAIDs, symptoms may improve, although the histologic findings of CDD may not be reversed.3,6 In one case report, a colonic stricture persisted endoscopically after NSAID withdrawal but resolved after a 20-week course of prednisone.7
Conclusion
Despite NSAID cessation, the patient in this case study presented a month later with an acute bowel obstruction. The bowel obstruction responded to conservative treatment, and the patient declined surgical intervention. The patient was readmitted again 4 months later with an acute bowel obstruction, and underwent an emergent colonic resection. The pathology report was consistent with CDD. In the 3 years since the resection, the patient has had no recurrences and continues to avoid all NSAIDs. She has refused a follow-up postresection colonoscopy.
1. Munipalle PC, Garud T, Light D. Diaphragmatic disease of the colon: systematic review. Colorectal Dis. 2013;15(9):1063-1069.
2. Ishihara M, Ohmiya N, Nakamura M, et al. Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther. 2014;40(5):538-547.
3. Keeling AN, Davis JL, Williams A, Sabharwal T, Adam A. Fluoroscopically guided balloon dilation of NSAID-induced colonic diaphragm. J Vasc Interv Radiol. 2007;18(8):1060-1062.
4. Yousfi MM, De Petris G, Leighton JA, et al. Diaphragm disease after use of nonsteroidal anti-inflammatory agents: first report of diagnosis with capsule endoscopy. J Clin Gastroenterol. 2004;38(8):686-691.
5. Pilgrim S, Velchuru V, Waters G, Tsiamis A, Lal R. Diaphragm disease and small bowel enteropathy due to nonsteroidal anti-inflammatory drugs: a surgical perspective. Colorectal Dis. 2011;13(4):463-466.
6. Munipalle PC, Little M, Garud T, Henderson D. NSAID-induced diaphragmatic disease of the colon. BMJ Case Rep. 2013;2013:pii:bcr2012008448.
7. Penner RM, Williams CN. Resolution of multiple severe nonsteroidal anti-inflammatory drug-induced colonic strictures with prednisone therapy: a case report and review of the literature. Can J Gastroenterol. 2003;17(8):497-500.
1. Munipalle PC, Garud T, Light D. Diaphragmatic disease of the colon: systematic review. Colorectal Dis. 2013;15(9):1063-1069.
2. Ishihara M, Ohmiya N, Nakamura M, et al. Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther. 2014;40(5):538-547.
3. Keeling AN, Davis JL, Williams A, Sabharwal T, Adam A. Fluoroscopically guided balloon dilation of NSAID-induced colonic diaphragm. J Vasc Interv Radiol. 2007;18(8):1060-1062.
4. Yousfi MM, De Petris G, Leighton JA, et al. Diaphragm disease after use of nonsteroidal anti-inflammatory agents: first report of diagnosis with capsule endoscopy. J Clin Gastroenterol. 2004;38(8):686-691.
5. Pilgrim S, Velchuru V, Waters G, Tsiamis A, Lal R. Diaphragm disease and small bowel enteropathy due to nonsteroidal anti-inflammatory drugs: a surgical perspective. Colorectal Dis. 2011;13(4):463-466.
6. Munipalle PC, Little M, Garud T, Henderson D. NSAID-induced diaphragmatic disease of the colon. BMJ Case Rep. 2013;2013:pii:bcr2012008448.
7. Penner RM, Williams CN. Resolution of multiple severe nonsteroidal anti-inflammatory drug-induced colonic strictures with prednisone therapy: a case report and review of the literature. Can J Gastroenterol. 2003;17(8):497-500.