User login
Chronic kidney disease (CKD) affects nearly 37 million people (11%) in the US and is a leading cause of death and morbidity. Due to their older age and higher prevalence of comorbid conditions, the prevalence of CKD among veterans is approximately 34% higher than in the general population and the fourth most common chronic disease diagnosed among US veterans.1,2 US veterans and those with prior military service (MS) may be at a particularly high risk for CKD and associated health care outcomes including increased hospitalization and death. The observed excess burden of CKD is not mirrored in the general population, and it is unclear whether prior MS confers a unique risk profile for CKD.
Current estimates of CKD burden among veterans or those with prior MS are widely variable and have been limited by unique regions, specific exposure profiles, or to single health care systems. As such, there remains a paucity of data examining CKD burden more broadly. We performed a study in the adult population of the US to quantify associations with the extent of CKD, enumerate temporal trends of CKD among those with prior MS, describe risk within subgroups, and compare heterogeneity of risk factors for CKD by MS.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a suite of nationally representative, cross-sectional surveys of the noninstitutionalized US population. It is conducted by the National Center for Health Statistics and uses a stratified, clustered probability design, with surveys carried out without interruption, collated, and made accessible to the public at 2-year intervals.3 The survey consists of a questionnaire, physical examination, and laboratory data.
The inclusion criteria for our study were age ≥ 20 years along with serum creatinine and urinary albumin-creatinine measurements. The following definitions were used for the study:
• CKD: Estimated glomerular filtration rate < 60 mL/min/1.73 m2 calibrated to isotope dilution mass spectrometry (IDMS).
• Traceable: Creatinine-based CKD Epidemiology Collaboration formula or urinary albumin-creatine ratio ≥ 30 mg/g.
• MS: Positive response to the questions “Did you ever serve in the Armed Forces of the United States?” (1999 to 2010) or “Have you ever served on active duty in the US Armed Forces, military Reserves, or National Guard?” (2011 to 2018).
• Diabetes: Self-reported history, medication for diabetes, or glycated hemoglobin ≥ 7%.
• Hypertension: Blood pressure ≥ 140/90 or ≥ 130/40 mm Hg in the presence of diabetes, medication for hypertension, cardiovascular disease, or CKD, myocardial infarction, cardiac failure, or cerebrovascular disease by self-report.2,3
Analysis
Primary sampling unit, stratum, and weight variables were employed throughout to generate parameter estimates that are generalizable to the US population.4,5 The χ2 test and logistic regression, respectively, were employed for comparison of proportions and estimation of odds ratios. R Version 4.1.2 was employed for data analysis.
Results
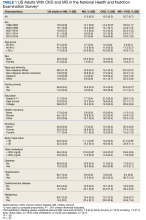
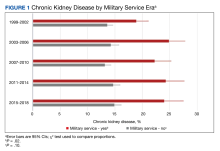
In the overall sample, the frequencies (95% standard error [SE]) of CKD and prior MS were 15.2% (0.3) and 11.5% (0.3) (Table 1). The proportion (SE) with CKD was significantly higher among those with prior MS vs the overall population: 22.7% (0.7) vs 15.2% (0.3) (P < .001). Significant associations with CKD were observed (P < .05) by age, sex, race and ethnicity, family poverty, school education, health insurance, smoking, body mass index, diabetes, hypertension, cardiovascular disease, and malignancy. Within those reporting prior MS, the proportion (SE) with CKD differed by era: 1999 to 2002, 18.9% (1.1); 2003 to 2006, 24.9% (1.5); 2007 to 2010, 22.3% (1.5); 2011 to 2014, 24.3% (1.7); and 2015 to 2018, 24.0% (1.8) (P = .02) (Figure 1).
Without covariate adjustment, prior MS was significantly associated with an increased risk of CKD (unadjusted odds ratio [OR], 1.78; 95% CI, 1.64-1.93; P < .05) (Table 2). Prior MS was significantly associated with CKD in the following subgroups: 2003 to 2006, 2011 to 2014, 2015 to 2018, age groups of 40 to 64 years and ≥ 65 years, male sex, non-Hispanic White and Hispanic ethnicity, school education of grade 0 to 11, and private or other health insurance. Additional comorbidities strongly associated with CKD included hypertension (OR, 6.37; 95% CI, 5.37-7.55), diabetes (OR, 4.16; 95% CI, 3.45-5.03), and cardiovascular disease (OR, 4.20; 95% CI, 3.57-4.95).
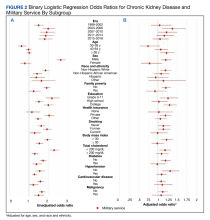
In the population reporting prior MS, the unadjusted OR of CKD vs 1999 to 2002 was greater for all other examined eras; with the greatest likelihood observed for the 2003 to 2006 era. Unadjusted ORs of CKD differed in groups with and without prior MS (P value for interaction < .05) for 2003 to 2006, those aged 40 to 64 years and ≥ 65 years, female sex, non-Hispanic African American and Hispanic race and ethnicity, family poverty, high school education, private health insurance, any smoking history, diabetes, hypertension, and cardiovascular disease (Figure 2A).
Following adjustment for age, sex, and race and ethnicity, MS was associated with a 17% higher likelihood of CKD (adjusted odds ratio [AOR], 1.17; 95% CI, 1.06-1.28; P < .01) (Table 3). Prior MS was significantly associated (P < .05) with CKD in the subgroups: age groups 40 to 64 years and ≥ 65 years, non-Hispanic African American, and body mass index ≥ 30. Among those with prior MS, comorbidities strongly associated with CKD in adjusted models included hypertension (AOR, 3.86; 95% CI, 3.18-4.69), diabetes (AOR, 3.05; 95% CI, 2.44-3.82), and cardiovascular disease (AOR, 2.51; 95% CI, 2.09-3.01). In the population with prior MS, the adjusted likelihood of CKD vs 1999 to 2002 was similar across all eras. Adjusted associations of CKD differed in groups with and without prior MS for age groups 40 to 64 years and ≥ 65 years, female sex, and family poverty (P < .05) (Figure 2B).
Discussion
We observed that prior MS was associated with CKD, all eras were associated with CKD in the subgroup with MS, and risk factors for CKD differed among many subgroups both with and without MS history, a finding that remained present in adjusted models. In addition, the finding of CKD was relatively common among those with prior MS (approximately 15%) and was most strongly associated with increasing age and comorbidities frequently associated with CKD.
Although many studies have demonstrated associations of US veteran status with various comorbidities, including hypertension, obesity, and diabetes, these studies often are limited to those both qualifying and receiving care within the US Department of Veterans Affairs (VA) health care system.6-9 The crude proportion of individuals reporting multiple chronic conditions, which included hypertension, diabetes, and weak or failing kidneys, was 49.7% for US veterans compared with 24.1% for nonveterans.2 Large-scale, nationally representative cohorts for use in this context have been limited by the heterogeneity of definitions of CKD applied with limited timeframes yielding variable estimates.1,10 Moreover, few studies have examined the clinical epidemiology of CKD more broadly in the US among those with prior MS. For example, a PubMed search on March 3, 2022, with the terms “epidemiology”, “military service”, and “chronic kidney disease” produced only 9 citations, one of which examined trends among a non-US cohort and quantifying disease burden another among adolescents.
Whether or not prior MS confers a unique risk profile for CKD is unknown. While our findings of an increased CKD burden among those reporting MS may partially reflect observed increases in baseline comorbidities, the observed excess CKD among those with MS remained across multiple categories even after adjustment for baseline demography. As several studies have demonstrated, enlistment into MS may select for a more diverse population; however those enlisted personnel may be of lower socioeconomic status and possibly at higher risk of CKD.11,12 Our findings of important differences in baseline determinants of health mirror this. The proportion of MS respondents with CKD vs CKD alone reporting a high school education or lower was higher (36.0% vs 21.8%) as well as among those with a history of family poverty (21.1% vs 18.0%).
Limitations
Our study has several limitations, including its cross-sectional study design, a lack of longitudinal data within individuals, and exclusion of institutionalized individuals. Limitations notwithstanding this study has several important aspects. As prior MS is highly variable, we were limited in our inability to stratify by service type or length of service. For example, veteran status is conferred to a “Reservist or member of the National Guard called to federal active duty or disabled from a disease or injury incurred or aggravated in line of duty or while in training status also qualify as a veteran” (13 CFR § 125.11). For the purposes of our study, prior MS would include all active-duty service (veterans) as well as reservists and National Guard members who have not been activated. This may be more representative of the overall effect of MS, as limitation to those receiving care within the VA may select for an older, more multimorbid population of patients, limiting generalizability.
In addition, more detailed information regarding service-related exposures and other service-connected conditions would allow for a more granular risk assessment by service type, era, and military conflict. Our finding of excess CKD burden among those with prior MS compared with the overall population is timely given the recent passage of the Promise to Address Comprehensive Toxics (PACT) Act. Exposure to and injury from Agent Orange—a known service-connected exposure associated with incident hypertension and diabetes—may be a significant contributor to CKD that may have a significant era effect. In addition, water contamination among those stationed in Camp Lejeune in North Carolina has notable genitourinary associations. Finally, burn pit exposures in more recent military conflicts may also have important associations with chronic disease, possibly including CKD. While similar attempts at the creation of large-scale US veteran cohorts have been limited by incomplete capture of creatinine, the large proportion of missing race data, and limited inclusion of additional markers of kidney disease, our use of a well-described, nationally representative survey along with standardized capture of clinical and laboratory elements mitigate the use of various societal or other codified definitions.1
Conclusions
Prior MS is associated with an increased risk of CKD overall and across several important subgroups. This finding was observed in various unadjusted and adjusted models and may constitute a unique risk profile of risk.
1. Ozieh MN, Gebregziabher M, Ward RC, Taber DJ, Egede LE. Creating a 13-year National Longitudinal Cohort of veterans with chronic kidney disease. BMC Nephrol. 2019;20(1):241. doi:10.1186/s12882-019-1430-y
2. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13.
3. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Survey. 2022. Accessed October 31, 2023. www.cdc.gov/nchs/nhanes/index.htm
4. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
5. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918-926. doi:10.1053/j.ajkd.2007.08.020
6. Smoley BA, Smith NL, Runkle GP. Hypertension in a population of active duty service members. J Am Board Fam Med. 2008;21(6):504-511. doi:10.3122/jabfm.2008.06.070182
7. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139. doi:10.1056/NEJMoa0808431
8. Smith TJ, Marriott BP, Dotson L, et al. Overweight and obesity in military personnel: sociodemographic predictors. Obesity (Silver Spring). 2012;20(7):1534-1538. doi:10.1038/oby.2012.25
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
11. Wang L, Elder GH, Jr., Spence NJ. Status configurations, military service and higher education. Soc Forces. 2012;91(2):397-422. doi:10.1093/sf/sos174
12. Zeng X, Liu J, Tao S, Hong HG, Li Y, Fu P. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72(4):270-279. doi:10.1136/jech-2017-209815
Chronic kidney disease (CKD) affects nearly 37 million people (11%) in the US and is a leading cause of death and morbidity. Due to their older age and higher prevalence of comorbid conditions, the prevalence of CKD among veterans is approximately 34% higher than in the general population and the fourth most common chronic disease diagnosed among US veterans.1,2 US veterans and those with prior military service (MS) may be at a particularly high risk for CKD and associated health care outcomes including increased hospitalization and death. The observed excess burden of CKD is not mirrored in the general population, and it is unclear whether prior MS confers a unique risk profile for CKD.
Current estimates of CKD burden among veterans or those with prior MS are widely variable and have been limited by unique regions, specific exposure profiles, or to single health care systems. As such, there remains a paucity of data examining CKD burden more broadly. We performed a study in the adult population of the US to quantify associations with the extent of CKD, enumerate temporal trends of CKD among those with prior MS, describe risk within subgroups, and compare heterogeneity of risk factors for CKD by MS.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a suite of nationally representative, cross-sectional surveys of the noninstitutionalized US population. It is conducted by the National Center for Health Statistics and uses a stratified, clustered probability design, with surveys carried out without interruption, collated, and made accessible to the public at 2-year intervals.3 The survey consists of a questionnaire, physical examination, and laboratory data.
The inclusion criteria for our study were age ≥ 20 years along with serum creatinine and urinary albumin-creatinine measurements. The following definitions were used for the study:
• CKD: Estimated glomerular filtration rate < 60 mL/min/1.73 m2 calibrated to isotope dilution mass spectrometry (IDMS).
• Traceable: Creatinine-based CKD Epidemiology Collaboration formula or urinary albumin-creatine ratio ≥ 30 mg/g.
• MS: Positive response to the questions “Did you ever serve in the Armed Forces of the United States?” (1999 to 2010) or “Have you ever served on active duty in the US Armed Forces, military Reserves, or National Guard?” (2011 to 2018).
• Diabetes: Self-reported history, medication for diabetes, or glycated hemoglobin ≥ 7%.
• Hypertension: Blood pressure ≥ 140/90 or ≥ 130/40 mm Hg in the presence of diabetes, medication for hypertension, cardiovascular disease, or CKD, myocardial infarction, cardiac failure, or cerebrovascular disease by self-report.2,3
Analysis
Primary sampling unit, stratum, and weight variables were employed throughout to generate parameter estimates that are generalizable to the US population.4,5 The χ2 test and logistic regression, respectively, were employed for comparison of proportions and estimation of odds ratios. R Version 4.1.2 was employed for data analysis.
Results
In the overall sample, the frequencies (95% standard error [SE]) of CKD and prior MS were 15.2% (0.3) and 11.5% (0.3) (Table 1). The proportion (SE) with CKD was significantly higher among those with prior MS vs the overall population: 22.7% (0.7) vs 15.2% (0.3) (P < .001). Significant associations with CKD were observed (P < .05) by age, sex, race and ethnicity, family poverty, school education, health insurance, smoking, body mass index, diabetes, hypertension, cardiovascular disease, and malignancy. Within those reporting prior MS, the proportion (SE) with CKD differed by era: 1999 to 2002, 18.9% (1.1); 2003 to 2006, 24.9% (1.5); 2007 to 2010, 22.3% (1.5); 2011 to 2014, 24.3% (1.7); and 2015 to 2018, 24.0% (1.8) (P = .02) (Figure 1).
Without covariate adjustment, prior MS was significantly associated with an increased risk of CKD (unadjusted odds ratio [OR], 1.78; 95% CI, 1.64-1.93; P < .05) (Table 2). Prior MS was significantly associated with CKD in the following subgroups: 2003 to 2006, 2011 to 2014, 2015 to 2018, age groups of 40 to 64 years and ≥ 65 years, male sex, non-Hispanic White and Hispanic ethnicity, school education of grade 0 to 11, and private or other health insurance. Additional comorbidities strongly associated with CKD included hypertension (OR, 6.37; 95% CI, 5.37-7.55), diabetes (OR, 4.16; 95% CI, 3.45-5.03), and cardiovascular disease (OR, 4.20; 95% CI, 3.57-4.95).
In the population reporting prior MS, the unadjusted OR of CKD vs 1999 to 2002 was greater for all other examined eras; with the greatest likelihood observed for the 2003 to 2006 era. Unadjusted ORs of CKD differed in groups with and without prior MS (P value for interaction < .05) for 2003 to 2006, those aged 40 to 64 years and ≥ 65 years, female sex, non-Hispanic African American and Hispanic race and ethnicity, family poverty, high school education, private health insurance, any smoking history, diabetes, hypertension, and cardiovascular disease (Figure 2A).
Following adjustment for age, sex, and race and ethnicity, MS was associated with a 17% higher likelihood of CKD (adjusted odds ratio [AOR], 1.17; 95% CI, 1.06-1.28; P < .01) (Table 3). Prior MS was significantly associated (P < .05) with CKD in the subgroups: age groups 40 to 64 years and ≥ 65 years, non-Hispanic African American, and body mass index ≥ 30. Among those with prior MS, comorbidities strongly associated with CKD in adjusted models included hypertension (AOR, 3.86; 95% CI, 3.18-4.69), diabetes (AOR, 3.05; 95% CI, 2.44-3.82), and cardiovascular disease (AOR, 2.51; 95% CI, 2.09-3.01). In the population with prior MS, the adjusted likelihood of CKD vs 1999 to 2002 was similar across all eras. Adjusted associations of CKD differed in groups with and without prior MS for age groups 40 to 64 years and ≥ 65 years, female sex, and family poverty (P < .05) (Figure 2B).
Discussion
We observed that prior MS was associated with CKD, all eras were associated with CKD in the subgroup with MS, and risk factors for CKD differed among many subgroups both with and without MS history, a finding that remained present in adjusted models. In addition, the finding of CKD was relatively common among those with prior MS (approximately 15%) and was most strongly associated with increasing age and comorbidities frequently associated with CKD.
Although many studies have demonstrated associations of US veteran status with various comorbidities, including hypertension, obesity, and diabetes, these studies often are limited to those both qualifying and receiving care within the US Department of Veterans Affairs (VA) health care system.6-9 The crude proportion of individuals reporting multiple chronic conditions, which included hypertension, diabetes, and weak or failing kidneys, was 49.7% for US veterans compared with 24.1% for nonveterans.2 Large-scale, nationally representative cohorts for use in this context have been limited by the heterogeneity of definitions of CKD applied with limited timeframes yielding variable estimates.1,10 Moreover, few studies have examined the clinical epidemiology of CKD more broadly in the US among those with prior MS. For example, a PubMed search on March 3, 2022, with the terms “epidemiology”, “military service”, and “chronic kidney disease” produced only 9 citations, one of which examined trends among a non-US cohort and quantifying disease burden another among adolescents.
Whether or not prior MS confers a unique risk profile for CKD is unknown. While our findings of an increased CKD burden among those reporting MS may partially reflect observed increases in baseline comorbidities, the observed excess CKD among those with MS remained across multiple categories even after adjustment for baseline demography. As several studies have demonstrated, enlistment into MS may select for a more diverse population; however those enlisted personnel may be of lower socioeconomic status and possibly at higher risk of CKD.11,12 Our findings of important differences in baseline determinants of health mirror this. The proportion of MS respondents with CKD vs CKD alone reporting a high school education or lower was higher (36.0% vs 21.8%) as well as among those with a history of family poverty (21.1% vs 18.0%).
Limitations
Our study has several limitations, including its cross-sectional study design, a lack of longitudinal data within individuals, and exclusion of institutionalized individuals. Limitations notwithstanding this study has several important aspects. As prior MS is highly variable, we were limited in our inability to stratify by service type or length of service. For example, veteran status is conferred to a “Reservist or member of the National Guard called to federal active duty or disabled from a disease or injury incurred or aggravated in line of duty or while in training status also qualify as a veteran” (13 CFR § 125.11). For the purposes of our study, prior MS would include all active-duty service (veterans) as well as reservists and National Guard members who have not been activated. This may be more representative of the overall effect of MS, as limitation to those receiving care within the VA may select for an older, more multimorbid population of patients, limiting generalizability.
In addition, more detailed information regarding service-related exposures and other service-connected conditions would allow for a more granular risk assessment by service type, era, and military conflict. Our finding of excess CKD burden among those with prior MS compared with the overall population is timely given the recent passage of the Promise to Address Comprehensive Toxics (PACT) Act. Exposure to and injury from Agent Orange—a known service-connected exposure associated with incident hypertension and diabetes—may be a significant contributor to CKD that may have a significant era effect. In addition, water contamination among those stationed in Camp Lejeune in North Carolina has notable genitourinary associations. Finally, burn pit exposures in more recent military conflicts may also have important associations with chronic disease, possibly including CKD. While similar attempts at the creation of large-scale US veteran cohorts have been limited by incomplete capture of creatinine, the large proportion of missing race data, and limited inclusion of additional markers of kidney disease, our use of a well-described, nationally representative survey along with standardized capture of clinical and laboratory elements mitigate the use of various societal or other codified definitions.1
Conclusions
Prior MS is associated with an increased risk of CKD overall and across several important subgroups. This finding was observed in various unadjusted and adjusted models and may constitute a unique risk profile of risk.
Chronic kidney disease (CKD) affects nearly 37 million people (11%) in the US and is a leading cause of death and morbidity. Due to their older age and higher prevalence of comorbid conditions, the prevalence of CKD among veterans is approximately 34% higher than in the general population and the fourth most common chronic disease diagnosed among US veterans.1,2 US veterans and those with prior military service (MS) may be at a particularly high risk for CKD and associated health care outcomes including increased hospitalization and death. The observed excess burden of CKD is not mirrored in the general population, and it is unclear whether prior MS confers a unique risk profile for CKD.
Current estimates of CKD burden among veterans or those with prior MS are widely variable and have been limited by unique regions, specific exposure profiles, or to single health care systems. As such, there remains a paucity of data examining CKD burden more broadly. We performed a study in the adult population of the US to quantify associations with the extent of CKD, enumerate temporal trends of CKD among those with prior MS, describe risk within subgroups, and compare heterogeneity of risk factors for CKD by MS.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a suite of nationally representative, cross-sectional surveys of the noninstitutionalized US population. It is conducted by the National Center for Health Statistics and uses a stratified, clustered probability design, with surveys carried out without interruption, collated, and made accessible to the public at 2-year intervals.3 The survey consists of a questionnaire, physical examination, and laboratory data.
The inclusion criteria for our study were age ≥ 20 years along with serum creatinine and urinary albumin-creatinine measurements. The following definitions were used for the study:
• CKD: Estimated glomerular filtration rate < 60 mL/min/1.73 m2 calibrated to isotope dilution mass spectrometry (IDMS).
• Traceable: Creatinine-based CKD Epidemiology Collaboration formula or urinary albumin-creatine ratio ≥ 30 mg/g.
• MS: Positive response to the questions “Did you ever serve in the Armed Forces of the United States?” (1999 to 2010) or “Have you ever served on active duty in the US Armed Forces, military Reserves, or National Guard?” (2011 to 2018).
• Diabetes: Self-reported history, medication for diabetes, or glycated hemoglobin ≥ 7%.
• Hypertension: Blood pressure ≥ 140/90 or ≥ 130/40 mm Hg in the presence of diabetes, medication for hypertension, cardiovascular disease, or CKD, myocardial infarction, cardiac failure, or cerebrovascular disease by self-report.2,3
Analysis
Primary sampling unit, stratum, and weight variables were employed throughout to generate parameter estimates that are generalizable to the US population.4,5 The χ2 test and logistic regression, respectively, were employed for comparison of proportions and estimation of odds ratios. R Version 4.1.2 was employed for data analysis.
Results
In the overall sample, the frequencies (95% standard error [SE]) of CKD and prior MS were 15.2% (0.3) and 11.5% (0.3) (Table 1). The proportion (SE) with CKD was significantly higher among those with prior MS vs the overall population: 22.7% (0.7) vs 15.2% (0.3) (P < .001). Significant associations with CKD were observed (P < .05) by age, sex, race and ethnicity, family poverty, school education, health insurance, smoking, body mass index, diabetes, hypertension, cardiovascular disease, and malignancy. Within those reporting prior MS, the proportion (SE) with CKD differed by era: 1999 to 2002, 18.9% (1.1); 2003 to 2006, 24.9% (1.5); 2007 to 2010, 22.3% (1.5); 2011 to 2014, 24.3% (1.7); and 2015 to 2018, 24.0% (1.8) (P = .02) (Figure 1).
Without covariate adjustment, prior MS was significantly associated with an increased risk of CKD (unadjusted odds ratio [OR], 1.78; 95% CI, 1.64-1.93; P < .05) (Table 2). Prior MS was significantly associated with CKD in the following subgroups: 2003 to 2006, 2011 to 2014, 2015 to 2018, age groups of 40 to 64 years and ≥ 65 years, male sex, non-Hispanic White and Hispanic ethnicity, school education of grade 0 to 11, and private or other health insurance. Additional comorbidities strongly associated with CKD included hypertension (OR, 6.37; 95% CI, 5.37-7.55), diabetes (OR, 4.16; 95% CI, 3.45-5.03), and cardiovascular disease (OR, 4.20; 95% CI, 3.57-4.95).
In the population reporting prior MS, the unadjusted OR of CKD vs 1999 to 2002 was greater for all other examined eras; with the greatest likelihood observed for the 2003 to 2006 era. Unadjusted ORs of CKD differed in groups with and without prior MS (P value for interaction < .05) for 2003 to 2006, those aged 40 to 64 years and ≥ 65 years, female sex, non-Hispanic African American and Hispanic race and ethnicity, family poverty, high school education, private health insurance, any smoking history, diabetes, hypertension, and cardiovascular disease (Figure 2A).
Following adjustment for age, sex, and race and ethnicity, MS was associated with a 17% higher likelihood of CKD (adjusted odds ratio [AOR], 1.17; 95% CI, 1.06-1.28; P < .01) (Table 3). Prior MS was significantly associated (P < .05) with CKD in the subgroups: age groups 40 to 64 years and ≥ 65 years, non-Hispanic African American, and body mass index ≥ 30. Among those with prior MS, comorbidities strongly associated with CKD in adjusted models included hypertension (AOR, 3.86; 95% CI, 3.18-4.69), diabetes (AOR, 3.05; 95% CI, 2.44-3.82), and cardiovascular disease (AOR, 2.51; 95% CI, 2.09-3.01). In the population with prior MS, the adjusted likelihood of CKD vs 1999 to 2002 was similar across all eras. Adjusted associations of CKD differed in groups with and without prior MS for age groups 40 to 64 years and ≥ 65 years, female sex, and family poverty (P < .05) (Figure 2B).
Discussion
We observed that prior MS was associated with CKD, all eras were associated with CKD in the subgroup with MS, and risk factors for CKD differed among many subgroups both with and without MS history, a finding that remained present in adjusted models. In addition, the finding of CKD was relatively common among those with prior MS (approximately 15%) and was most strongly associated with increasing age and comorbidities frequently associated with CKD.
Although many studies have demonstrated associations of US veteran status with various comorbidities, including hypertension, obesity, and diabetes, these studies often are limited to those both qualifying and receiving care within the US Department of Veterans Affairs (VA) health care system.6-9 The crude proportion of individuals reporting multiple chronic conditions, which included hypertension, diabetes, and weak or failing kidneys, was 49.7% for US veterans compared with 24.1% for nonveterans.2 Large-scale, nationally representative cohorts for use in this context have been limited by the heterogeneity of definitions of CKD applied with limited timeframes yielding variable estimates.1,10 Moreover, few studies have examined the clinical epidemiology of CKD more broadly in the US among those with prior MS. For example, a PubMed search on March 3, 2022, with the terms “epidemiology”, “military service”, and “chronic kidney disease” produced only 9 citations, one of which examined trends among a non-US cohort and quantifying disease burden another among adolescents.
Whether or not prior MS confers a unique risk profile for CKD is unknown. While our findings of an increased CKD burden among those reporting MS may partially reflect observed increases in baseline comorbidities, the observed excess CKD among those with MS remained across multiple categories even after adjustment for baseline demography. As several studies have demonstrated, enlistment into MS may select for a more diverse population; however those enlisted personnel may be of lower socioeconomic status and possibly at higher risk of CKD.11,12 Our findings of important differences in baseline determinants of health mirror this. The proportion of MS respondents with CKD vs CKD alone reporting a high school education or lower was higher (36.0% vs 21.8%) as well as among those with a history of family poverty (21.1% vs 18.0%).
Limitations
Our study has several limitations, including its cross-sectional study design, a lack of longitudinal data within individuals, and exclusion of institutionalized individuals. Limitations notwithstanding this study has several important aspects. As prior MS is highly variable, we were limited in our inability to stratify by service type or length of service. For example, veteran status is conferred to a “Reservist or member of the National Guard called to federal active duty or disabled from a disease or injury incurred or aggravated in line of duty or while in training status also qualify as a veteran” (13 CFR § 125.11). For the purposes of our study, prior MS would include all active-duty service (veterans) as well as reservists and National Guard members who have not been activated. This may be more representative of the overall effect of MS, as limitation to those receiving care within the VA may select for an older, more multimorbid population of patients, limiting generalizability.
In addition, more detailed information regarding service-related exposures and other service-connected conditions would allow for a more granular risk assessment by service type, era, and military conflict. Our finding of excess CKD burden among those with prior MS compared with the overall population is timely given the recent passage of the Promise to Address Comprehensive Toxics (PACT) Act. Exposure to and injury from Agent Orange—a known service-connected exposure associated with incident hypertension and diabetes—may be a significant contributor to CKD that may have a significant era effect. In addition, water contamination among those stationed in Camp Lejeune in North Carolina has notable genitourinary associations. Finally, burn pit exposures in more recent military conflicts may also have important associations with chronic disease, possibly including CKD. While similar attempts at the creation of large-scale US veteran cohorts have been limited by incomplete capture of creatinine, the large proportion of missing race data, and limited inclusion of additional markers of kidney disease, our use of a well-described, nationally representative survey along with standardized capture of clinical and laboratory elements mitigate the use of various societal or other codified definitions.1
Conclusions
Prior MS is associated with an increased risk of CKD overall and across several important subgroups. This finding was observed in various unadjusted and adjusted models and may constitute a unique risk profile of risk.
1. Ozieh MN, Gebregziabher M, Ward RC, Taber DJ, Egede LE. Creating a 13-year National Longitudinal Cohort of veterans with chronic kidney disease. BMC Nephrol. 2019;20(1):241. doi:10.1186/s12882-019-1430-y
2. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13.
3. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Survey. 2022. Accessed October 31, 2023. www.cdc.gov/nchs/nhanes/index.htm
4. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
5. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918-926. doi:10.1053/j.ajkd.2007.08.020
6. Smoley BA, Smith NL, Runkle GP. Hypertension in a population of active duty service members. J Am Board Fam Med. 2008;21(6):504-511. doi:10.3122/jabfm.2008.06.070182
7. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139. doi:10.1056/NEJMoa0808431
8. Smith TJ, Marriott BP, Dotson L, et al. Overweight and obesity in military personnel: sociodemographic predictors. Obesity (Silver Spring). 2012;20(7):1534-1538. doi:10.1038/oby.2012.25
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
11. Wang L, Elder GH, Jr., Spence NJ. Status configurations, military service and higher education. Soc Forces. 2012;91(2):397-422. doi:10.1093/sf/sos174
12. Zeng X, Liu J, Tao S, Hong HG, Li Y, Fu P. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72(4):270-279. doi:10.1136/jech-2017-209815
1. Ozieh MN, Gebregziabher M, Ward RC, Taber DJ, Egede LE. Creating a 13-year National Longitudinal Cohort of veterans with chronic kidney disease. BMC Nephrol. 2019;20(1):241. doi:10.1186/s12882-019-1430-y
2. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13.
3. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Survey. 2022. Accessed October 31, 2023. www.cdc.gov/nchs/nhanes/index.htm
4. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
5. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918-926. doi:10.1053/j.ajkd.2007.08.020
6. Smoley BA, Smith NL, Runkle GP. Hypertension in a population of active duty service members. J Am Board Fam Med. 2008;21(6):504-511. doi:10.3122/jabfm.2008.06.070182
7. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139. doi:10.1056/NEJMoa0808431
8. Smith TJ, Marriott BP, Dotson L, et al. Overweight and obesity in military personnel: sociodemographic predictors. Obesity (Silver Spring). 2012;20(7):1534-1538. doi:10.1038/oby.2012.25
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
11. Wang L, Elder GH, Jr., Spence NJ. Status configurations, military service and higher education. Soc Forces. 2012;91(2):397-422. doi:10.1093/sf/sos174
12. Zeng X, Liu J, Tao S, Hong HG, Li Y, Fu P. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72(4):270-279. doi:10.1136/jech-2017-209815