User login
The author is a consultant to Merck & Co., Inc., GlaxoSmithKline, and Roche Molecular Diagnostics.
New data enhance our knowledge in two critical areas previously covered in this update: the human papillomavirus (HPV) vaccine and HPV DNA testing for cervical cancer screening.
Among findings published in 2007:
- results of phase-3 trials of the quadrivalent and bivalent HPV vaccines, which confirm the remarkable efficacy seen in phase-2 trials among women not previously exposed to the vaccine-targeted HPV types
- three large randomized cervical cancer screening trials from Canada, Sweden, and the Netherlands, which confirm the superiority of cervical cancer screening programs that add HPV DNA testing to cytology in women 30 years and older
- the much-anticipated update of consensus guidelines on the management of women with abnormal cervical cancer screening tests.
Efficacy of HPV vaccine approaches 100% in targeted population
Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927.
Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943.
These trials, referred to as FUTURE I and II, were multicenter, multicountry, double-blinded, placebo-controlled trials of the quadrivalent vaccine (Gardasil) that enrolled women 15 to 26 years of age (TABLE).1 Participants were followed for an average of 3 years after receiving the first of the series of three vaccinations. The efficacy of the vaccine at preventing high-grade neoplasia associated with vaccine-targeted HPV types (HPV 6, 11, 16, and 18) was 98% and 100% in the two trials among the “per-protocol,” susceptible population. That population was defined as women who had no evidence of exposure to the targeted HPV types, according to serologic or HPV DNA testing during the first 7 months of the trials, and who received all three vaccinations. This population is a good indicator of how the vaccine will work in adolescents who are not yet sexually active (TABLE).
Efficacy is high in key phase-3 trials of the HPV vaccine
| AUTHORS | HPV TYPES TARGETED | WOMEN (N) | FOLLOW-UP | ENDPOINT | VACCINE EFFICACY (%)* |
|---|---|---|---|---|---|
| Garland et al (FUTURE I) (2007) | 6, 11, 16, 18 | 4,499 | 3 years | CIN 2+ and adenocarcinoma in situ | 100 (95% CI, 94–100) |
| FUTURE II Study Group (2007) | 6, 11, 16, 18 | 12,167 | 3 years | CIN 2+ and adenocarcinoma in situ | 98 (95% CI, 86–100) |
| Joura et al1 (2007) | 6, 11, 16, 18 | 15,596 | 3 years | Vulvar intraepithelial neoplasia 2+ and vaginal intraepithelial neoplasia 2+ | 100 (95% CI, 72–100) |
| Paavonen et al (2007) | 16, 18 | 15,626 | 15 months | CIN 2+ | 90 (95% CI, 53–99) |
| * In women naïve to vaccine-targeted HPV types by serology and HPV DNA testing. | |||||
| CI, confidence interval; CIN, cervical intraepithelial neoplasia. | |||||
The vaccine was less effective in the “intention-to-treat” population that included all women enrolled in the study regardless of HPV status. At 3 years, the vaccine reduced high-grade neoplasia associated with vaccine-targeted HPV types in this population by only 29% and 50% in the two trials.
When these results were published, some experts expressed concern and questioned the benefit of the vaccines.2 There is no reason for concern, however, because lower short-term efficacy in the intention-to-treat population was expected. Because the current generation of HPV vaccines does not have a measurable therapeutic effect, vaccination will not prevent cervical intraepithelial neoplasia (CIN) in women who are already infected with vaccine-targeted HPV types; nor will it cause regression of CIN lesions that are already present when the woman is vaccinated.
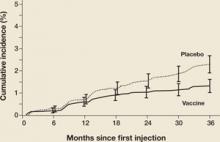
There were a number of women already infected with vaccine-targeted HPV types at enrollment in the “intention-to-treat” population. Some of these women developed CIN 2,3 associated with vaccine-targeted HPV strains during the first 18 months of the trial (FIGURE 1). However, with longer follow-up, the cumulative number of cases of CIN 2,3 plateaued in vaccinated women, whereas it continued to rise in the placebo arm. Thus, with longer follow-up, vaccine efficacy will improve. Therefore, both the American College of Obstetricians and Gynecologists and the Advisory Committee on Immunization Practices recommend that all sexually active adolescents and young women be vaccinated through 26 years of age.
Figure 1 Cases of CIN 2,3 eventually plateau in vaccinated women
The graph charts the efficacy of the quadrivalent HPV vaccine in preventing CIN 2,3 in the intention-to-treat population of a phase-3 efficacy trial.
SOURCE: Garland et al. Copyright © 2008 Massachusetts Medical Society. All rights reserved.
Bivalent vaccine was 90.4% effective against CIN 2,3
Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170.
This interim analysis of a phase-3 double-blind, placebo-controlled trial of the bivalent HPV vaccine (Cervarix), which targets HPV types 16 and 18, also was published last year. It enrolled more than 18,000 women 15 to 25 years old who had a mean length of follow-up of 15 months. The vaccine was 90.4% effective against CIN 2,3 associated with the targeted strains (types 16 and 18), the primary endpoint of the trial (TABLE). There was no significant difference in safety outcome between vaccine and placebo recipients.
This trial is ongoing, with final results expected in approximately 2 years. Based on interim findings, the vaccine has been approved for use in a number of countries, including all 27 European Union nations, and the manufacturer of the vaccine has filed an application for approval with the US Food and Drug Administration (FDA).
Screening is more effective when HPV testing is included—or used alone
Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:1764–1772.
Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–1597.
Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588.
These major studies compared cytology alone with HPV DNA testing for high-risk strains (16, 18, 31, 33, 35, 45, 51, 52, 56, 58, 59, 68) and found HPV testing—with or without cytology—to be superior to cytology alone.
In a trial from the Netherlands, Bulkmans and colleagues randomly assigned more than 17,000 women 29 years and older to cytologic screening only or a combination of cytology and HPV DNA testing. After 5 years of follow-up, all women were rescreened using both tests. The baseline screen including a combination of cytology and HPV DNA testing identified 70% more CIN 3 lesions and cancers than did cytology alone. More important, during the subsequent round of screening, CIN 3 lesions and cancers decreased by 55% in the group initially screened with both tests.
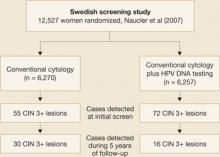
Naucler and associates had similar results in a prospective Swedish trial that randomized women to screening by cytology alone or a combination of cytology and HPV DNA testing. During the initial round of screening, 31% more CIN 3 lesions and cancers were detected in the group screened with both tests (FIGURE 2). In subsequent rounds of screening, 47% fewer CIN 3 lesions or cancers were identified in this group.
Figure 2 Screening protocol that includes HPV DNA testing is superior, large trial confirmsTaken together, these two prospective studies clearly demonstrate that the addition of HPV DNA testing to cytology increases detection of high-grade lesions and reduces the incidence of high-grade neoplasia and cancers detected subsequently.
HPV testing is more sensitive, only slightly less specific, than cytology
In a cross-sectional study from Canada, Mayrand and colleagues compared HPV DNA testing and cytology during a single round of screening in more than 10,000 women. The findings were consistent with those of previous studies showing HPV DNA testing to be significantly more sensitive but somewhat less specific than cytology.3
The sensitivity of HPV testing for CIN 2,3 was 95% (95% confidence interval [CI], 84–100), compared with 55% (95% CI, 34–77) for cytology. Specificity of HPV DNA testing and cytology was 94% and 97%, respectively. When the two tests were used together, sensitivity was 100% and specificity was 93%.
New consensus guidelines clarify screening in special populations
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355.
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340–345.
The 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests clarify management of special populations such as adolescents, postmenopausal women, and patients with cervical adenocarcinoma in situ. Although the 2001 guidelines were widely adopted in the United States as the standard for managing women with abnormal screening tests—more than 500,000 copies were downloaded from Web site of the American Society for Colposcopy and Cervical Pathology (ASCCP)4—it became apparent after their implementation in a variety of clinical settings that some clarification of the guidelines was needed.
In adolescents, treat abnormalities conservatively
A major theme of the 2006 guidelines is a more conservative approach to adolescent patients (ages 13 to 20 years). Although this population has a very low risk of developing invasive cervical cancer, women 15 to 19 years of age are very likely to be diagnosed with minor cytologic abnormalities such as atypical squamous cells of undetermined significance (ASC-US) and low-grade squamous intraepithelial lesions (LSIL), owing to the very high prevalence of anogenital HPV infection in this age group.
Because most anogenital HPV infections will spontaneously clear, minor cytologic abnormalities are usually of little consequence in adolescents.
Therefore, the 2006 consensus guidelines discourage the use of colposcopy in adolescents who have ASC-US and LSIL. Instead, these patients should be followed with annual repeat cytology and referred to colposcopy only when a high-grade cytologic abnormality is identified or when a low-grade cytologic abnormality persists for 24 months.
HPV testing most informative in older women
The new guidelines expand the clinical indications for HPV DNA testing and provide recommendations for managing different combinations of cytology and HPV test results when screening women 30 years and older. For example, they emphasize the use of HPV DNA testing in postmenopausal women because recent studies clearly demonstrate that the prevalence of high-risk HPV DNA positivity is lower in postmenopausal women with ASC-US or LSIL than in younger women.
Use only FDA-approved HPV tests
With the expanded indications for HPV DNA testing, the new guidelines take pains to point out that HPV test methods that have not been approved by the FDA may not produce findings consistent with approved methods. This is a very important point because many laboratories have started using unapproved testing methods. Although these methods have been validated internally by the laboratories, they have not been through the rigorous evaluation required for FDA approval. The new guidelines therefore state: “Appropriate use of these guidelines requires that laboratories utilize only HPV tests that have been analytically and clinically validated with proven acceptable reproducibility, clinical sensitivity, specificity, and positive and negative predictive values for cervical cancer and verified precancer (CIN 2,3), as documented by FDA approval and/or publication in peer-reviewed scientific literature.”
The guidelines are accessible online at the ASCCP Web site at www.asccp.org/consensus/cytological.shtml.
1. Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
2. Sawaya GF. Smith-McCune K. HPV vaccination—more answers, more questions. N Engl J Med. 2007;356:1991-1993.
3. Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
4. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB. Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA .2002;287:2120-2129.
The author is a consultant to Merck & Co., Inc., GlaxoSmithKline, and Roche Molecular Diagnostics.
New data enhance our knowledge in two critical areas previously covered in this update: the human papillomavirus (HPV) vaccine and HPV DNA testing for cervical cancer screening.
Among findings published in 2007:
- results of phase-3 trials of the quadrivalent and bivalent HPV vaccines, which confirm the remarkable efficacy seen in phase-2 trials among women not previously exposed to the vaccine-targeted HPV types
- three large randomized cervical cancer screening trials from Canada, Sweden, and the Netherlands, which confirm the superiority of cervical cancer screening programs that add HPV DNA testing to cytology in women 30 years and older
- the much-anticipated update of consensus guidelines on the management of women with abnormal cervical cancer screening tests.
Efficacy of HPV vaccine approaches 100% in targeted population
Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927.
Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943.
These trials, referred to as FUTURE I and II, were multicenter, multicountry, double-blinded, placebo-controlled trials of the quadrivalent vaccine (Gardasil) that enrolled women 15 to 26 years of age (TABLE).1 Participants were followed for an average of 3 years after receiving the first of the series of three vaccinations. The efficacy of the vaccine at preventing high-grade neoplasia associated with vaccine-targeted HPV types (HPV 6, 11, 16, and 18) was 98% and 100% in the two trials among the “per-protocol,” susceptible population. That population was defined as women who had no evidence of exposure to the targeted HPV types, according to serologic or HPV DNA testing during the first 7 months of the trials, and who received all three vaccinations. This population is a good indicator of how the vaccine will work in adolescents who are not yet sexually active (TABLE).
Efficacy is high in key phase-3 trials of the HPV vaccine
| AUTHORS | HPV TYPES TARGETED | WOMEN (N) | FOLLOW-UP | ENDPOINT | VACCINE EFFICACY (%)* |
|---|---|---|---|---|---|
| Garland et al (FUTURE I) (2007) | 6, 11, 16, 18 | 4,499 | 3 years | CIN 2+ and adenocarcinoma in situ | 100 (95% CI, 94–100) |
| FUTURE II Study Group (2007) | 6, 11, 16, 18 | 12,167 | 3 years | CIN 2+ and adenocarcinoma in situ | 98 (95% CI, 86–100) |
| Joura et al1 (2007) | 6, 11, 16, 18 | 15,596 | 3 years | Vulvar intraepithelial neoplasia 2+ and vaginal intraepithelial neoplasia 2+ | 100 (95% CI, 72–100) |
| Paavonen et al (2007) | 16, 18 | 15,626 | 15 months | CIN 2+ | 90 (95% CI, 53–99) |
| * In women naïve to vaccine-targeted HPV types by serology and HPV DNA testing. | |||||
| CI, confidence interval; CIN, cervical intraepithelial neoplasia. | |||||
The vaccine was less effective in the “intention-to-treat” population that included all women enrolled in the study regardless of HPV status. At 3 years, the vaccine reduced high-grade neoplasia associated with vaccine-targeted HPV types in this population by only 29% and 50% in the two trials.
When these results were published, some experts expressed concern and questioned the benefit of the vaccines.2 There is no reason for concern, however, because lower short-term efficacy in the intention-to-treat population was expected. Because the current generation of HPV vaccines does not have a measurable therapeutic effect, vaccination will not prevent cervical intraepithelial neoplasia (CIN) in women who are already infected with vaccine-targeted HPV types; nor will it cause regression of CIN lesions that are already present when the woman is vaccinated.
There were a number of women already infected with vaccine-targeted HPV types at enrollment in the “intention-to-treat” population. Some of these women developed CIN 2,3 associated with vaccine-targeted HPV strains during the first 18 months of the trial (FIGURE 1). However, with longer follow-up, the cumulative number of cases of CIN 2,3 plateaued in vaccinated women, whereas it continued to rise in the placebo arm. Thus, with longer follow-up, vaccine efficacy will improve. Therefore, both the American College of Obstetricians and Gynecologists and the Advisory Committee on Immunization Practices recommend that all sexually active adolescents and young women be vaccinated through 26 years of age.
Figure 1 Cases of CIN 2,3 eventually plateau in vaccinated women
The graph charts the efficacy of the quadrivalent HPV vaccine in preventing CIN 2,3 in the intention-to-treat population of a phase-3 efficacy trial.
SOURCE: Garland et al. Copyright © 2008 Massachusetts Medical Society. All rights reserved.
Bivalent vaccine was 90.4% effective against CIN 2,3
Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170.
This interim analysis of a phase-3 double-blind, placebo-controlled trial of the bivalent HPV vaccine (Cervarix), which targets HPV types 16 and 18, also was published last year. It enrolled more than 18,000 women 15 to 25 years old who had a mean length of follow-up of 15 months. The vaccine was 90.4% effective against CIN 2,3 associated with the targeted strains (types 16 and 18), the primary endpoint of the trial (TABLE). There was no significant difference in safety outcome between vaccine and placebo recipients.
This trial is ongoing, with final results expected in approximately 2 years. Based on interim findings, the vaccine has been approved for use in a number of countries, including all 27 European Union nations, and the manufacturer of the vaccine has filed an application for approval with the US Food and Drug Administration (FDA).
Screening is more effective when HPV testing is included—or used alone
Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:1764–1772.
Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–1597.
Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588.
These major studies compared cytology alone with HPV DNA testing for high-risk strains (16, 18, 31, 33, 35, 45, 51, 52, 56, 58, 59, 68) and found HPV testing—with or without cytology—to be superior to cytology alone.
In a trial from the Netherlands, Bulkmans and colleagues randomly assigned more than 17,000 women 29 years and older to cytologic screening only or a combination of cytology and HPV DNA testing. After 5 years of follow-up, all women were rescreened using both tests. The baseline screen including a combination of cytology and HPV DNA testing identified 70% more CIN 3 lesions and cancers than did cytology alone. More important, during the subsequent round of screening, CIN 3 lesions and cancers decreased by 55% in the group initially screened with both tests.
Naucler and associates had similar results in a prospective Swedish trial that randomized women to screening by cytology alone or a combination of cytology and HPV DNA testing. During the initial round of screening, 31% more CIN 3 lesions and cancers were detected in the group screened with both tests (FIGURE 2). In subsequent rounds of screening, 47% fewer CIN 3 lesions or cancers were identified in this group.
Figure 2 Screening protocol that includes HPV DNA testing is superior, large trial confirmsTaken together, these two prospective studies clearly demonstrate that the addition of HPV DNA testing to cytology increases detection of high-grade lesions and reduces the incidence of high-grade neoplasia and cancers detected subsequently.
HPV testing is more sensitive, only slightly less specific, than cytology
In a cross-sectional study from Canada, Mayrand and colleagues compared HPV DNA testing and cytology during a single round of screening in more than 10,000 women. The findings were consistent with those of previous studies showing HPV DNA testing to be significantly more sensitive but somewhat less specific than cytology.3
The sensitivity of HPV testing for CIN 2,3 was 95% (95% confidence interval [CI], 84–100), compared with 55% (95% CI, 34–77) for cytology. Specificity of HPV DNA testing and cytology was 94% and 97%, respectively. When the two tests were used together, sensitivity was 100% and specificity was 93%.
New consensus guidelines clarify screening in special populations
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355.
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340–345.
The 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests clarify management of special populations such as adolescents, postmenopausal women, and patients with cervical adenocarcinoma in situ. Although the 2001 guidelines were widely adopted in the United States as the standard for managing women with abnormal screening tests—more than 500,000 copies were downloaded from Web site of the American Society for Colposcopy and Cervical Pathology (ASCCP)4—it became apparent after their implementation in a variety of clinical settings that some clarification of the guidelines was needed.
In adolescents, treat abnormalities conservatively
A major theme of the 2006 guidelines is a more conservative approach to adolescent patients (ages 13 to 20 years). Although this population has a very low risk of developing invasive cervical cancer, women 15 to 19 years of age are very likely to be diagnosed with minor cytologic abnormalities such as atypical squamous cells of undetermined significance (ASC-US) and low-grade squamous intraepithelial lesions (LSIL), owing to the very high prevalence of anogenital HPV infection in this age group.
Because most anogenital HPV infections will spontaneously clear, minor cytologic abnormalities are usually of little consequence in adolescents.
Therefore, the 2006 consensus guidelines discourage the use of colposcopy in adolescents who have ASC-US and LSIL. Instead, these patients should be followed with annual repeat cytology and referred to colposcopy only when a high-grade cytologic abnormality is identified or when a low-grade cytologic abnormality persists for 24 months.
HPV testing most informative in older women
The new guidelines expand the clinical indications for HPV DNA testing and provide recommendations for managing different combinations of cytology and HPV test results when screening women 30 years and older. For example, they emphasize the use of HPV DNA testing in postmenopausal women because recent studies clearly demonstrate that the prevalence of high-risk HPV DNA positivity is lower in postmenopausal women with ASC-US or LSIL than in younger women.
Use only FDA-approved HPV tests
With the expanded indications for HPV DNA testing, the new guidelines take pains to point out that HPV test methods that have not been approved by the FDA may not produce findings consistent with approved methods. This is a very important point because many laboratories have started using unapproved testing methods. Although these methods have been validated internally by the laboratories, they have not been through the rigorous evaluation required for FDA approval. The new guidelines therefore state: “Appropriate use of these guidelines requires that laboratories utilize only HPV tests that have been analytically and clinically validated with proven acceptable reproducibility, clinical sensitivity, specificity, and positive and negative predictive values for cervical cancer and verified precancer (CIN 2,3), as documented by FDA approval and/or publication in peer-reviewed scientific literature.”
The guidelines are accessible online at the ASCCP Web site at www.asccp.org/consensus/cytological.shtml.
The author is a consultant to Merck & Co., Inc., GlaxoSmithKline, and Roche Molecular Diagnostics.
New data enhance our knowledge in two critical areas previously covered in this update: the human papillomavirus (HPV) vaccine and HPV DNA testing for cervical cancer screening.
Among findings published in 2007:
- results of phase-3 trials of the quadrivalent and bivalent HPV vaccines, which confirm the remarkable efficacy seen in phase-2 trials among women not previously exposed to the vaccine-targeted HPV types
- three large randomized cervical cancer screening trials from Canada, Sweden, and the Netherlands, which confirm the superiority of cervical cancer screening programs that add HPV DNA testing to cytology in women 30 years and older
- the much-anticipated update of consensus guidelines on the management of women with abnormal cervical cancer screening tests.
Efficacy of HPV vaccine approaches 100% in targeted population
Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927.
Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943.
These trials, referred to as FUTURE I and II, were multicenter, multicountry, double-blinded, placebo-controlled trials of the quadrivalent vaccine (Gardasil) that enrolled women 15 to 26 years of age (TABLE).1 Participants were followed for an average of 3 years after receiving the first of the series of three vaccinations. The efficacy of the vaccine at preventing high-grade neoplasia associated with vaccine-targeted HPV types (HPV 6, 11, 16, and 18) was 98% and 100% in the two trials among the “per-protocol,” susceptible population. That population was defined as women who had no evidence of exposure to the targeted HPV types, according to serologic or HPV DNA testing during the first 7 months of the trials, and who received all three vaccinations. This population is a good indicator of how the vaccine will work in adolescents who are not yet sexually active (TABLE).
Efficacy is high in key phase-3 trials of the HPV vaccine
| AUTHORS | HPV TYPES TARGETED | WOMEN (N) | FOLLOW-UP | ENDPOINT | VACCINE EFFICACY (%)* |
|---|---|---|---|---|---|
| Garland et al (FUTURE I) (2007) | 6, 11, 16, 18 | 4,499 | 3 years | CIN 2+ and adenocarcinoma in situ | 100 (95% CI, 94–100) |
| FUTURE II Study Group (2007) | 6, 11, 16, 18 | 12,167 | 3 years | CIN 2+ and adenocarcinoma in situ | 98 (95% CI, 86–100) |
| Joura et al1 (2007) | 6, 11, 16, 18 | 15,596 | 3 years | Vulvar intraepithelial neoplasia 2+ and vaginal intraepithelial neoplasia 2+ | 100 (95% CI, 72–100) |
| Paavonen et al (2007) | 16, 18 | 15,626 | 15 months | CIN 2+ | 90 (95% CI, 53–99) |
| * In women naïve to vaccine-targeted HPV types by serology and HPV DNA testing. | |||||
| CI, confidence interval; CIN, cervical intraepithelial neoplasia. | |||||
The vaccine was less effective in the “intention-to-treat” population that included all women enrolled in the study regardless of HPV status. At 3 years, the vaccine reduced high-grade neoplasia associated with vaccine-targeted HPV types in this population by only 29% and 50% in the two trials.
When these results were published, some experts expressed concern and questioned the benefit of the vaccines.2 There is no reason for concern, however, because lower short-term efficacy in the intention-to-treat population was expected. Because the current generation of HPV vaccines does not have a measurable therapeutic effect, vaccination will not prevent cervical intraepithelial neoplasia (CIN) in women who are already infected with vaccine-targeted HPV types; nor will it cause regression of CIN lesions that are already present when the woman is vaccinated.
There were a number of women already infected with vaccine-targeted HPV types at enrollment in the “intention-to-treat” population. Some of these women developed CIN 2,3 associated with vaccine-targeted HPV strains during the first 18 months of the trial (FIGURE 1). However, with longer follow-up, the cumulative number of cases of CIN 2,3 plateaued in vaccinated women, whereas it continued to rise in the placebo arm. Thus, with longer follow-up, vaccine efficacy will improve. Therefore, both the American College of Obstetricians and Gynecologists and the Advisory Committee on Immunization Practices recommend that all sexually active adolescents and young women be vaccinated through 26 years of age.
Figure 1 Cases of CIN 2,3 eventually plateau in vaccinated women
The graph charts the efficacy of the quadrivalent HPV vaccine in preventing CIN 2,3 in the intention-to-treat population of a phase-3 efficacy trial.
SOURCE: Garland et al. Copyright © 2008 Massachusetts Medical Society. All rights reserved.
Bivalent vaccine was 90.4% effective against CIN 2,3
Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170.
This interim analysis of a phase-3 double-blind, placebo-controlled trial of the bivalent HPV vaccine (Cervarix), which targets HPV types 16 and 18, also was published last year. It enrolled more than 18,000 women 15 to 25 years old who had a mean length of follow-up of 15 months. The vaccine was 90.4% effective against CIN 2,3 associated with the targeted strains (types 16 and 18), the primary endpoint of the trial (TABLE). There was no significant difference in safety outcome between vaccine and placebo recipients.
This trial is ongoing, with final results expected in approximately 2 years. Based on interim findings, the vaccine has been approved for use in a number of countries, including all 27 European Union nations, and the manufacturer of the vaccine has filed an application for approval with the US Food and Drug Administration (FDA).
Screening is more effective when HPV testing is included—or used alone
Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:1764–1772.
Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–1597.
Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588.
These major studies compared cytology alone with HPV DNA testing for high-risk strains (16, 18, 31, 33, 35, 45, 51, 52, 56, 58, 59, 68) and found HPV testing—with or without cytology—to be superior to cytology alone.
In a trial from the Netherlands, Bulkmans and colleagues randomly assigned more than 17,000 women 29 years and older to cytologic screening only or a combination of cytology and HPV DNA testing. After 5 years of follow-up, all women were rescreened using both tests. The baseline screen including a combination of cytology and HPV DNA testing identified 70% more CIN 3 lesions and cancers than did cytology alone. More important, during the subsequent round of screening, CIN 3 lesions and cancers decreased by 55% in the group initially screened with both tests.
Naucler and associates had similar results in a prospective Swedish trial that randomized women to screening by cytology alone or a combination of cytology and HPV DNA testing. During the initial round of screening, 31% more CIN 3 lesions and cancers were detected in the group screened with both tests (FIGURE 2). In subsequent rounds of screening, 47% fewer CIN 3 lesions or cancers were identified in this group.
Figure 2 Screening protocol that includes HPV DNA testing is superior, large trial confirmsTaken together, these two prospective studies clearly demonstrate that the addition of HPV DNA testing to cytology increases detection of high-grade lesions and reduces the incidence of high-grade neoplasia and cancers detected subsequently.
HPV testing is more sensitive, only slightly less specific, than cytology
In a cross-sectional study from Canada, Mayrand and colleagues compared HPV DNA testing and cytology during a single round of screening in more than 10,000 women. The findings were consistent with those of previous studies showing HPV DNA testing to be significantly more sensitive but somewhat less specific than cytology.3
The sensitivity of HPV testing for CIN 2,3 was 95% (95% confidence interval [CI], 84–100), compared with 55% (95% CI, 34–77) for cytology. Specificity of HPV DNA testing and cytology was 94% and 97%, respectively. When the two tests were used together, sensitivity was 100% and specificity was 93%.
New consensus guidelines clarify screening in special populations
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355.
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340–345.
The 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests clarify management of special populations such as adolescents, postmenopausal women, and patients with cervical adenocarcinoma in situ. Although the 2001 guidelines were widely adopted in the United States as the standard for managing women with abnormal screening tests—more than 500,000 copies were downloaded from Web site of the American Society for Colposcopy and Cervical Pathology (ASCCP)4—it became apparent after their implementation in a variety of clinical settings that some clarification of the guidelines was needed.
In adolescents, treat abnormalities conservatively
A major theme of the 2006 guidelines is a more conservative approach to adolescent patients (ages 13 to 20 years). Although this population has a very low risk of developing invasive cervical cancer, women 15 to 19 years of age are very likely to be diagnosed with minor cytologic abnormalities such as atypical squamous cells of undetermined significance (ASC-US) and low-grade squamous intraepithelial lesions (LSIL), owing to the very high prevalence of anogenital HPV infection in this age group.
Because most anogenital HPV infections will spontaneously clear, minor cytologic abnormalities are usually of little consequence in adolescents.
Therefore, the 2006 consensus guidelines discourage the use of colposcopy in adolescents who have ASC-US and LSIL. Instead, these patients should be followed with annual repeat cytology and referred to colposcopy only when a high-grade cytologic abnormality is identified or when a low-grade cytologic abnormality persists for 24 months.
HPV testing most informative in older women
The new guidelines expand the clinical indications for HPV DNA testing and provide recommendations for managing different combinations of cytology and HPV test results when screening women 30 years and older. For example, they emphasize the use of HPV DNA testing in postmenopausal women because recent studies clearly demonstrate that the prevalence of high-risk HPV DNA positivity is lower in postmenopausal women with ASC-US or LSIL than in younger women.
Use only FDA-approved HPV tests
With the expanded indications for HPV DNA testing, the new guidelines take pains to point out that HPV test methods that have not been approved by the FDA may not produce findings consistent with approved methods. This is a very important point because many laboratories have started using unapproved testing methods. Although these methods have been validated internally by the laboratories, they have not been through the rigorous evaluation required for FDA approval. The new guidelines therefore state: “Appropriate use of these guidelines requires that laboratories utilize only HPV tests that have been analytically and clinically validated with proven acceptable reproducibility, clinical sensitivity, specificity, and positive and negative predictive values for cervical cancer and verified precancer (CIN 2,3), as documented by FDA approval and/or publication in peer-reviewed scientific literature.”
The guidelines are accessible online at the ASCCP Web site at www.asccp.org/consensus/cytological.shtml.
1. Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
2. Sawaya GF. Smith-McCune K. HPV vaccination—more answers, more questions. N Engl J Med. 2007;356:1991-1993.
3. Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
4. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB. Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA .2002;287:2120-2129.
1. Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
2. Sawaya GF. Smith-McCune K. HPV vaccination—more answers, more questions. N Engl J Med. 2007;356:1991-1993.
3. Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
4. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB. Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA .2002;287:2120-2129.