User login
Over the past few decades, it has become increasingly clear that cannabis use can increase the risk of developing a psychotic disorder and worsen the course of existing schizophrenia in a dose-dependent fashion.1-3 Beyond psychosis, although many patients with mental illness use cannabis for recreational purposes or as purported “self-medication,” currently available evidence suggests that marijuana is more likely to represent a harm than a benefit for psychiatric disorders4 (Box4-8). Our current state of knowledge therefore suggests that psychiatrists should caution their patients against using cannabis and prioritize interventions to reduce or discontinue use, especially among those with psychotic disorders.
Box
Data from California in 2006—a decade after the state’s legalization of “medical marijuana”—revealed that 23% of patients in a sample enrolled in medical marijuana clinics were receiving cannabis to treat a mental disorder.5 That was a striking statistic given the dearth of evidence to support a benefit of cannabis for psychiatric conditions at the time, leaving clinicians who provided the necessary recommendations to obtain medical marijuana largely unable to give informed consent about the risks and benefits, much less recommendations about specific products, routes of administration, or dosing. In 2019, we know considerably more about the interaction between cannabinoids and mental health, but research findings thus far warrant more caution than enthusiasm, with one recent review concluding that “whenever an association is observed between cannabis use and psychiatric disorders, the relationship is generally an adverse one.”4
Some critics have argued that the medical marijuana industry represents little more than a front for recreational use. In California and other states that have legalized recreational use, that claim has been rendered all but moot, although the public remains curious about the potential health benefits of cannabinoids and will likely continue to look to clinicians for advice. For those seeking guidance from evidence-based research, the existing state of knowledge can seem like a “Wild West” of anecdotal subjective reports, biased opinions, and uncontrolled clinical studies. Cannabis remains a Schedule I drug at the federal level, and quality clinical research has been limited to a relatively modest number of randomized controlled trials (RCTs), mostly involving FDA-approved cannabinoids rather than smoked cannabis. Randomized controlled trials that have involved smoked marijuana have generally involved low-potency delta-9-tetrahydrocannabinol (THC) cannabis that may not reflect the same therapeutic and adverse effects of the increasingly high potency cannabis now available on the street and in dispensaries.
In psychiatry, a few RCTs are underway exploring cannabis as a viable treatment for mental disorders (eg, posttraumatic stress disorder), but none have yet been completed or published. At best, retrospective studies to date have failed to support a consistent benefit of cannabis for any psychiatric disorder and at worst increasingly suggest a negative impact on psychotic, mood, and anxiety disorders.4,6 Meanwhile, synthetic cannabinoid receptor agonists (eg, “Spice” products) have come to represent a clear public health risk, with both medical and psychiatric toxicity.7
A more cautiously optimistic case for the therapeutic potential of cannabinoids in psychiatry could be made for cannabidiol (CBD), which may possess anxiolytic, antipsychotic, and neuroprotective properties.8 Based on its purported health benefits, it is possible that CBD may even gain widespread popularity as a food supplement. Because a pharmaceutically-manufactured form of CBD was recently FDA-approved for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, off-label prescribing of CBD for psychiatric disorders can be anticipated. While there is not yet sufficient evidence about risks and benefits to justify CBD being recommended broadly in psychiatry, that same informational vacuum has not stopped eager patients from seeking approval for cannabis, and some physicians from providing it.
Despite that conclusion, because cannabis is classified as a Schedule I drug by the US Drug Enforcement Agency, clinical research investigating the risks and benefits of cannabis has been limited. It therefore remains possible that cannabis, or individual cannabinoids such as cannabidiol (CBD), may yet find a therapeutic niche in psychiatry. This article reviews evidence on CBD for the treatment of schizophrenia.
Cannabinergic drugs as potential antipsychotics
Although the bulk of evidence indicates a harmful effect of cannabis in individuals with or at risk for psychosis, there have been a few published cases of schizophrenia improving with dronabinol, an FDA-approved, synthetic form of delta-9-tetrahydrocannabinol (THC).9,10 THC is the constituent of cannabis that produces euphoric effects. These provocative findings have not been replicated in controlled clinical trials, but suggest at least the theoretical possibility of idiosyncratic benefits from THC for some individuals within the psychotic spectrum.
Still, given that most available evidence supports that THC has a harmful effect on psychosis and psychosis risk, researchers have instead performed randomized controlled trials (RCTs) to investigate a possible therapeutic role for medications that oppose the agonist effects of THC at cannabinoid type 1 (CB1) receptors. To date, 2 RCTs comparing rimonabant, a CB1 inverse agonist, with placebo (PLB) in patients with schizophrenia have failed to demonstrate any benefit for psychotic symptoms or cognitive deficits.11,12 A third trial examining rimonabant for people diagnosed with schizophrenia who were overweight found significant benefits for anxiety and depressive symptoms, but none for positive symptoms or the primary outcome of weight loss.13 While these results are discouraging, the role of THC in precipitating psychosis suggests that novel agents opposing the actions of THC on the cannabinoid system could have antipsychotic properties.14
Cannabidiol: An antipsychotic medication?
In contrast to THC, CBD has minimal euphorigenic properties and has recently been heralded in the popular press as a “miracle drug” with benefits for medical and psychiatric disorders alike.15 It has even been speculated that it could become a popular food supplement.16 In 2018, the FDA gave full approval to a pharmaceutically manufactured form of CBD (brand name: Epidiolex) as a novel treatment for 2 rare and severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,17 based on RCTs supporting its efficacy for these often refractory and life-threatening conditions.18-20
In psychiatry, there have not yet been enough robust clinical studies to support broad therapeutic claims for CBD as a treatment for any mental disorder.21 However, there is growing evidence that CBD has potential as an antipsychotic medication. In 1995, the first case report was published describing the efficacy of CBD, 1,500 mg/d, as standalone therapy in a single individual with schizophrenia.22 In 2006, the same research group followed up with a case series in which only 1 out of 3 patients with treatment-refractory schizophrenia improved with flexible dosing of CBD to a maximum dose of 1,280 mg/d.23
There have been 3 published RCTs exploring the efficacy of CBD in schizophrenia (Table24-26). The first study, published in 2012, included 39 adults with schizophrenia who were randomized to 800 mg/d of CBD or amisulpride (AMS), a second-generation antipsychotic that is popular in Europe but is not available in the United States.24 Over 4 weeks of randomized treatment, CBD resulted in as much improvement in overall symptoms and positive symptoms as AMS, and improvement of negative symptoms was significantly greater with CBD. Compared with patients treated with antipsychotic medication, patients who were treated with CBD had fewer extrapyramidal symptoms, less weight gain, and less prolactin elevation. This initial trial suggests that CBD might be as efficacious in schizophrenia as antipsychotic medication, without its burdensome adverse effects. However, this is the only RCT of CBD monotherapy published to date.
Continue to: Two other recently published RCTs...
Two other recently published RCTs compared CBD with PLB as add-on therapy to antipsychotics. McGuire et al25 compared CBD, 1,000 mg/d, to PLB over 6 weeks in 88 patients with schizophrenia. Positive symptom improvement was statistically greater with CBD than with PLB, although the magnitude of clinical change was modest (using the Positive and Negative Syndrome Scale [PANSS] positive symptom subscale: −3.2 points for CBD vs −1.7 points for PLB). Changes in PANSS total score and subscales for general and negative symptoms were not significantly different between treatment groups. There was also no significant difference in overall change in neurocognitive symptoms, although post-hoc analysis revealed significantly greater improvement in motor speed for patients treated with CBD. More than twice the number of patients treated with CBD were rated as “much improved” by the Clinical Global Impressions scale compared with patients treated with PLB, but this was not a statistically significant finding, and most patients experienced only “minimal” or “no improvement.” In terms of adverse events, there were no significant differences between patients in the CBD and PLB groups. Although this study is technically “positive” for CBD and suggests minimal adverse effects, it is not clear whether the statistically significant positive symptom improvements (+1.5 PANSS points for CBD over PLB) were clinically significant.
The most recently published placebo-controlled RCT of CBD as add-on therapy to antipsychotic medication included 36 patients with schizophrenia treated over 6 weeks.26 In this study, there was no benefit of CBD, 600 mg/d, on any PANSS score outcome (total, general, positive, or negative symptoms). For the primary outcome of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, there were no significant drug × time effects, and post-hoc analyses showed that only patients treated with PLB improved with time. Sedation was more common among patients treated with CBD compared with PLB.
Making sense of the data
There have been mixed results from the few case reports and 3 RCTs of patients with schizophrenia who were treated with CBD. How can we resolve these disparate findings? A few possible interpretations of the data that warrant clarification through additional research include:
Dosing. In the first case report with positive results, CBD was dosed at 1,500 mg/d,22 whereas in the subsequent case series with mixed results, the maximum allowable dose of CBD was 1,280 mg/d.23 Likewise, in the RCTs, positive results were found when CBD was dosed at 800 to 1,000 mg/d,24,25 but not at 600 mg/d.26 The efficacy of CBD for schizophrenia might depend on higher doses.
Treatment resistance. In the second case series in which only 1 out of 3 patients responded to treatment with CBD,23 the patients had demonstrated previous nonresponse to at least 2 first-generation antipsychotics (FGAs) and risperidone, 6 mg/d. In the RCTs, all patients were antipsychotic-responsive.24-26 Cannabidiol may not be as effective for patients with treatment-refractory schizophrenia as it is for patients with schizophrenia who respond to antipsychotics.
Continue to: Clinical stability
Clinical stability. Within the RCTs, the greatest response was observed in the study that enrolled patients who were hospitalized with acute symptoms of schizophrenia.23 In the 2 studies that found either modest or no benefit with CBD, the patients had been stabilized on antipsychotic medications prior to randomization. Cannabidiol may offer limited benefit as add-on therapy to patients who have already responded to antipsychotic treatment, where there is “less room” for additional improvement.
Monotherapy. Both the case reports22,23 and the RCT with the most robust positive findings24 involved treatment with CBD as monotherapy. For some patients with schizophrenia, CBD might be effective as standalone therapy as an alternative to antipsychotics that is better tolerated. Adding CBD to antipsychotic therapy might be redundant and therefore less effective.
Answering questions about CBD
Cannabidiol is becoming increasingly popular for its purported health benefits. The mixed results of the few studies published on CBD for schizophrenia place clinicians in a difficult position when attempting to answer questions about how cannabinoids might fit into treatment of patients with psychosis. Consider the following:
Is cannabis helpful for patients with schizophrenia? No. Aside from the few case reports suggesting that FDA-approved THC (dronabinol) can improve symptoms in some patients,9,10 most of the evidence from anecdotal reports and both experimental and observational studies indicate that cannabis, THC, and synthetic cannabinoids have a harmful effect in patients with or at risk for psychosis.1-3
If you are considering recommending some form of cannabis to patients with schizophrenia, what kind should you recommend? Recommending or encouraging cannabis use for patients with psychosis is ill-advised. Although certain types of cannabis might contain more THC (eg, Cannabis indica vs Cannabis sativa) or variable amounts of CBD, in general the amount of CBD in whole leaf cannabis is minimal, with the ratio of THC to CBD increasingly significantly over the past decade.3,27 Most forms of cannabis should therefore be avoided by individuals with or at risk for psychotic disorders.
Continue to: What about CBD oil and other CBD products sold in dispensaries?
What about CBD oil and other CBD products sold in dispensaries? Cannabidiol is increasingly available in various forms based on its ability to be designated as a legal hemp product (containing <0.3% THC) at the federal level or as a cannabinoid in states where cannabis is legal. However, several studies have now shown that cannabis products sold online or in dispensaries are often labeled inaccurately, with both under- and over-reporting of THC and CBD content.28-30 Some CBD products have been found to have almost no CBD at all.29,30 The unreliability of product labeling makes it difficult to predict the effects of CBD products that are not subject to FDA purity standards for medications or dietary supplements. It also raises questions about the sources of CBD and the reliability of dosing in the studies discussed above.
Why might CBD work as an antipsychotic? Although CBD has minimal affinity for cannabinoid receptors, it appears to act as a partial agonist of dopamine D2 receptors and an agonist at 5-HT1A receptors, with overall effects that decrease mesolimbic dopamine activity.31,32 In addition, CBD increases the availability of the endogenous cannabinoid anandamide, which may have antipsychotic properties.14,33
Now that the FDA has approved CBD manufactured by a pharmaceutical company, should it be prescribed “off-label” for patients with schizophrenia? This is the “million dollar question,” with insufficient evidence to provide a clear answer. It should now be possible to prescribe FDA-approved CBD for off-label purposes, including the treatment of schizophrenia and other psychiatric disorders. No doubt, some clinicians are already doing so. This will predictably yield more anecdotal evidence about efficacy and adverse effects in the future, but there is not yet adequate evidence to support an FDA indication for CBD in schizophrenia. Additional studies of CBD for schizophrenia are ongoing.
Bottom Line
Cannabidiol (CBD) is becoming increasingly popular based on its purported health benefits, but the evidence supporting a therapeutic role in psychiatry is preliminary at best. Although CBD is now available by prescription as an FDA-approved drug for the treatment of 2 rare forms of epilepsy, its benefits in patients with schizophrenia are uncertain based on mixed results in clinical trials.
Related Resources
- Clinicaltrials.gov. Studies of “cannabidiol” and “schizophrenia.” U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Schizophrenia&term=cannabidiol.
- Grinspoon P. Cannabidiol (CBD) – what we know and what we don’t. Harvard Health Blog. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-wedont-2018082414476. Published August 24, 2018.
Drug Brand Names
Cannabidiol • Epidiolex
Dronabinol • Marinol
Risperidone • Risperdal
1. Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: what the evidence says. Current Psychiatry. 2011;10(9):49-58.
2. Radhakrishan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
3. Pierre JM. Risks of increasingly potent cannabis: joint effects of potency and frequency. Current Psychiatry. 2016;16(2):14-20.
4. Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-255.
5. Nunberg H, Kilmer B, Pacula RL, et al. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011;4(1):1.
6. Wilkinson ST, Radhakrishnan, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
7. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus. 2016;38(3):344-366.
8. Crippa JA, Guimarães FS, Campos A, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009.
9. Schwarz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
10. Schwarz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
11. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975-984.
12. Boggs DL, Kelly DL, McMahon RP, et al. Rimonabant for neurocognition in schizophrenia: a 16-week double blind placebo controlled trial. Schizophr Res. 2012;134(2-3):207-210.
13. Kelly DL, Gorelick DA, Conley RR, et al. Effects of cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: a randomized, double-blind, pilot study. J Clin Psychopharmacol. 2011;31(1):86-91.
14. Leweke FM, Mueller JK, Lange B, et al. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604-612.
15. Halperin A. What is CBD? The ‘miracle’ cannabis compound that doesn’t get you high. The Guardian. https://www.theguardian.com/society/2018/may/28/what-is-cbd-cannabidiol-cannabis-medical-uses. Published May 28, 2018. Accessed April 3, 2019.
16. Pierre J. Coca, cola, and cannabis: psychoactive drugs as beverages. Psychology Today (blog) Psych Unseen. https://www.psychologytoday.com/us/blog/psych-unseen/201810/coca-cola-and-cannabis-psychoactive-drugs-beverages. Published October 1, 2018. Accessed April 3, 2019.
17. U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Published June 25, 2018. Accessed April 3, 2019.
18. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
19. Thiele EA, March ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096.
20. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
21. Khoury JM, Neves MCLD, Rogue MAV, et al. Is there a role of cannabidiol in psychiatry? World J Biol Psychiatry. 2017:1-16.
22. Zuardi AW, Morais SL, Guimares FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
23. Zuardi AW, Hallak JEC, Dursun SM. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
24. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15.
25. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
26. Boggs DL, Surti I, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol. 2018;235(7):1923-1932.
27. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016; 79(7):613-619.
28. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2492.
29. Ruth AC, Gryniewicz-Ruzicka CM, Trehy ML, et al. Consistency of label claims of internet-purchased hemp oil and cannabis products as determined using IMS and LC-MS: a marketplace study. J Reg Sci. 2016;3:1-6.
30. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
31. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. doi: 10.1038/tp.2016.195.
32. Renard J, Norris C, Rushlow W, et al. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;157-165.
33. Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res. 2016;176(2-3):281-290.
Over the past few decades, it has become increasingly clear that cannabis use can increase the risk of developing a psychotic disorder and worsen the course of existing schizophrenia in a dose-dependent fashion.1-3 Beyond psychosis, although many patients with mental illness use cannabis for recreational purposes or as purported “self-medication,” currently available evidence suggests that marijuana is more likely to represent a harm than a benefit for psychiatric disorders4 (Box4-8). Our current state of knowledge therefore suggests that psychiatrists should caution their patients against using cannabis and prioritize interventions to reduce or discontinue use, especially among those with psychotic disorders.
Box
Data from California in 2006—a decade after the state’s legalization of “medical marijuana”—revealed that 23% of patients in a sample enrolled in medical marijuana clinics were receiving cannabis to treat a mental disorder.5 That was a striking statistic given the dearth of evidence to support a benefit of cannabis for psychiatric conditions at the time, leaving clinicians who provided the necessary recommendations to obtain medical marijuana largely unable to give informed consent about the risks and benefits, much less recommendations about specific products, routes of administration, or dosing. In 2019, we know considerably more about the interaction between cannabinoids and mental health, but research findings thus far warrant more caution than enthusiasm, with one recent review concluding that “whenever an association is observed between cannabis use and psychiatric disorders, the relationship is generally an adverse one.”4
Some critics have argued that the medical marijuana industry represents little more than a front for recreational use. In California and other states that have legalized recreational use, that claim has been rendered all but moot, although the public remains curious about the potential health benefits of cannabinoids and will likely continue to look to clinicians for advice. For those seeking guidance from evidence-based research, the existing state of knowledge can seem like a “Wild West” of anecdotal subjective reports, biased opinions, and uncontrolled clinical studies. Cannabis remains a Schedule I drug at the federal level, and quality clinical research has been limited to a relatively modest number of randomized controlled trials (RCTs), mostly involving FDA-approved cannabinoids rather than smoked cannabis. Randomized controlled trials that have involved smoked marijuana have generally involved low-potency delta-9-tetrahydrocannabinol (THC) cannabis that may not reflect the same therapeutic and adverse effects of the increasingly high potency cannabis now available on the street and in dispensaries.
In psychiatry, a few RCTs are underway exploring cannabis as a viable treatment for mental disorders (eg, posttraumatic stress disorder), but none have yet been completed or published. At best, retrospective studies to date have failed to support a consistent benefit of cannabis for any psychiatric disorder and at worst increasingly suggest a negative impact on psychotic, mood, and anxiety disorders.4,6 Meanwhile, synthetic cannabinoid receptor agonists (eg, “Spice” products) have come to represent a clear public health risk, with both medical and psychiatric toxicity.7
A more cautiously optimistic case for the therapeutic potential of cannabinoids in psychiatry could be made for cannabidiol (CBD), which may possess anxiolytic, antipsychotic, and neuroprotective properties.8 Based on its purported health benefits, it is possible that CBD may even gain widespread popularity as a food supplement. Because a pharmaceutically-manufactured form of CBD was recently FDA-approved for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, off-label prescribing of CBD for psychiatric disorders can be anticipated. While there is not yet sufficient evidence about risks and benefits to justify CBD being recommended broadly in psychiatry, that same informational vacuum has not stopped eager patients from seeking approval for cannabis, and some physicians from providing it.
Despite that conclusion, because cannabis is classified as a Schedule I drug by the US Drug Enforcement Agency, clinical research investigating the risks and benefits of cannabis has been limited. It therefore remains possible that cannabis, or individual cannabinoids such as cannabidiol (CBD), may yet find a therapeutic niche in psychiatry. This article reviews evidence on CBD for the treatment of schizophrenia.
Cannabinergic drugs as potential antipsychotics
Although the bulk of evidence indicates a harmful effect of cannabis in individuals with or at risk for psychosis, there have been a few published cases of schizophrenia improving with dronabinol, an FDA-approved, synthetic form of delta-9-tetrahydrocannabinol (THC).9,10 THC is the constituent of cannabis that produces euphoric effects. These provocative findings have not been replicated in controlled clinical trials, but suggest at least the theoretical possibility of idiosyncratic benefits from THC for some individuals within the psychotic spectrum.
Still, given that most available evidence supports that THC has a harmful effect on psychosis and psychosis risk, researchers have instead performed randomized controlled trials (RCTs) to investigate a possible therapeutic role for medications that oppose the agonist effects of THC at cannabinoid type 1 (CB1) receptors. To date, 2 RCTs comparing rimonabant, a CB1 inverse agonist, with placebo (PLB) in patients with schizophrenia have failed to demonstrate any benefit for psychotic symptoms or cognitive deficits.11,12 A third trial examining rimonabant for people diagnosed with schizophrenia who were overweight found significant benefits for anxiety and depressive symptoms, but none for positive symptoms or the primary outcome of weight loss.13 While these results are discouraging, the role of THC in precipitating psychosis suggests that novel agents opposing the actions of THC on the cannabinoid system could have antipsychotic properties.14
Cannabidiol: An antipsychotic medication?
In contrast to THC, CBD has minimal euphorigenic properties and has recently been heralded in the popular press as a “miracle drug” with benefits for medical and psychiatric disorders alike.15 It has even been speculated that it could become a popular food supplement.16 In 2018, the FDA gave full approval to a pharmaceutically manufactured form of CBD (brand name: Epidiolex) as a novel treatment for 2 rare and severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,17 based on RCTs supporting its efficacy for these often refractory and life-threatening conditions.18-20
In psychiatry, there have not yet been enough robust clinical studies to support broad therapeutic claims for CBD as a treatment for any mental disorder.21 However, there is growing evidence that CBD has potential as an antipsychotic medication. In 1995, the first case report was published describing the efficacy of CBD, 1,500 mg/d, as standalone therapy in a single individual with schizophrenia.22 In 2006, the same research group followed up with a case series in which only 1 out of 3 patients with treatment-refractory schizophrenia improved with flexible dosing of CBD to a maximum dose of 1,280 mg/d.23
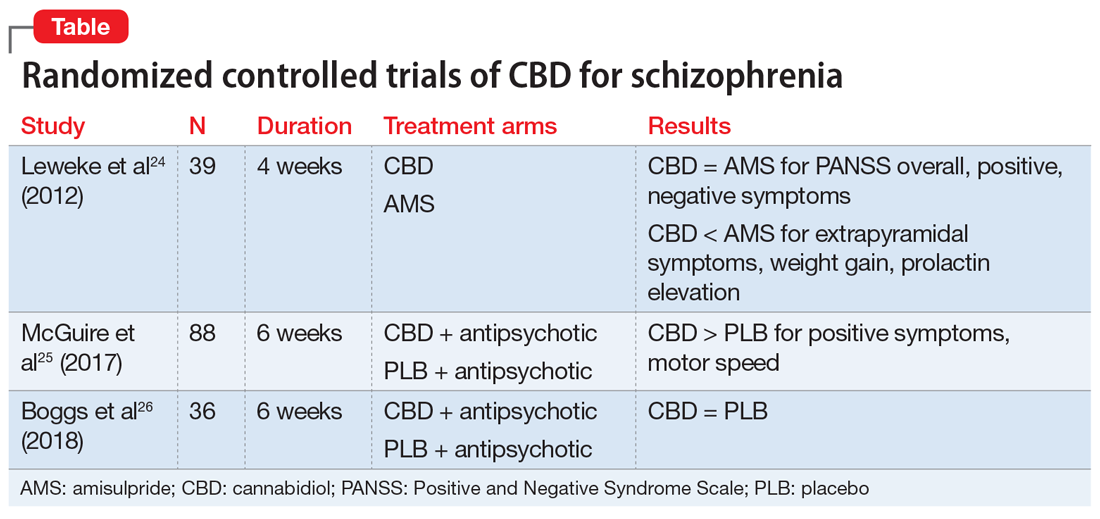
There have been 3 published RCTs exploring the efficacy of CBD in schizophrenia (Table24-26). The first study, published in 2012, included 39 adults with schizophrenia who were randomized to 800 mg/d of CBD or amisulpride (AMS), a second-generation antipsychotic that is popular in Europe but is not available in the United States.24 Over 4 weeks of randomized treatment, CBD resulted in as much improvement in overall symptoms and positive symptoms as AMS, and improvement of negative symptoms was significantly greater with CBD. Compared with patients treated with antipsychotic medication, patients who were treated with CBD had fewer extrapyramidal symptoms, less weight gain, and less prolactin elevation. This initial trial suggests that CBD might be as efficacious in schizophrenia as antipsychotic medication, without its burdensome adverse effects. However, this is the only RCT of CBD monotherapy published to date.
Continue to: Two other recently published RCTs...
Two other recently published RCTs compared CBD with PLB as add-on therapy to antipsychotics. McGuire et al25 compared CBD, 1,000 mg/d, to PLB over 6 weeks in 88 patients with schizophrenia. Positive symptom improvement was statistically greater with CBD than with PLB, although the magnitude of clinical change was modest (using the Positive and Negative Syndrome Scale [PANSS] positive symptom subscale: −3.2 points for CBD vs −1.7 points for PLB). Changes in PANSS total score and subscales for general and negative symptoms were not significantly different between treatment groups. There was also no significant difference in overall change in neurocognitive symptoms, although post-hoc analysis revealed significantly greater improvement in motor speed for patients treated with CBD. More than twice the number of patients treated with CBD were rated as “much improved” by the Clinical Global Impressions scale compared with patients treated with PLB, but this was not a statistically significant finding, and most patients experienced only “minimal” or “no improvement.” In terms of adverse events, there were no significant differences between patients in the CBD and PLB groups. Although this study is technically “positive” for CBD and suggests minimal adverse effects, it is not clear whether the statistically significant positive symptom improvements (+1.5 PANSS points for CBD over PLB) were clinically significant.
The most recently published placebo-controlled RCT of CBD as add-on therapy to antipsychotic medication included 36 patients with schizophrenia treated over 6 weeks.26 In this study, there was no benefit of CBD, 600 mg/d, on any PANSS score outcome (total, general, positive, or negative symptoms). For the primary outcome of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, there were no significant drug × time effects, and post-hoc analyses showed that only patients treated with PLB improved with time. Sedation was more common among patients treated with CBD compared with PLB.
Making sense of the data
There have been mixed results from the few case reports and 3 RCTs of patients with schizophrenia who were treated with CBD. How can we resolve these disparate findings? A few possible interpretations of the data that warrant clarification through additional research include:
Dosing. In the first case report with positive results, CBD was dosed at 1,500 mg/d,22 whereas in the subsequent case series with mixed results, the maximum allowable dose of CBD was 1,280 mg/d.23 Likewise, in the RCTs, positive results were found when CBD was dosed at 800 to 1,000 mg/d,24,25 but not at 600 mg/d.26 The efficacy of CBD for schizophrenia might depend on higher doses.
Treatment resistance. In the second case series in which only 1 out of 3 patients responded to treatment with CBD,23 the patients had demonstrated previous nonresponse to at least 2 first-generation antipsychotics (FGAs) and risperidone, 6 mg/d. In the RCTs, all patients were antipsychotic-responsive.24-26 Cannabidiol may not be as effective for patients with treatment-refractory schizophrenia as it is for patients with schizophrenia who respond to antipsychotics.
Continue to: Clinical stability
Clinical stability. Within the RCTs, the greatest response was observed in the study that enrolled patients who were hospitalized with acute symptoms of schizophrenia.23 In the 2 studies that found either modest or no benefit with CBD, the patients had been stabilized on antipsychotic medications prior to randomization. Cannabidiol may offer limited benefit as add-on therapy to patients who have already responded to antipsychotic treatment, where there is “less room” for additional improvement.
Monotherapy. Both the case reports22,23 and the RCT with the most robust positive findings24 involved treatment with CBD as monotherapy. For some patients with schizophrenia, CBD might be effective as standalone therapy as an alternative to antipsychotics that is better tolerated. Adding CBD to antipsychotic therapy might be redundant and therefore less effective.
Answering questions about CBD
Cannabidiol is becoming increasingly popular for its purported health benefits. The mixed results of the few studies published on CBD for schizophrenia place clinicians in a difficult position when attempting to answer questions about how cannabinoids might fit into treatment of patients with psychosis. Consider the following:
Is cannabis helpful for patients with schizophrenia? No. Aside from the few case reports suggesting that FDA-approved THC (dronabinol) can improve symptoms in some patients,9,10 most of the evidence from anecdotal reports and both experimental and observational studies indicate that cannabis, THC, and synthetic cannabinoids have a harmful effect in patients with or at risk for psychosis.1-3
If you are considering recommending some form of cannabis to patients with schizophrenia, what kind should you recommend? Recommending or encouraging cannabis use for patients with psychosis is ill-advised. Although certain types of cannabis might contain more THC (eg, Cannabis indica vs Cannabis sativa) or variable amounts of CBD, in general the amount of CBD in whole leaf cannabis is minimal, with the ratio of THC to CBD increasingly significantly over the past decade.3,27 Most forms of cannabis should therefore be avoided by individuals with or at risk for psychotic disorders.
Continue to: What about CBD oil and other CBD products sold in dispensaries?
What about CBD oil and other CBD products sold in dispensaries? Cannabidiol is increasingly available in various forms based on its ability to be designated as a legal hemp product (containing <0.3% THC) at the federal level or as a cannabinoid in states where cannabis is legal. However, several studies have now shown that cannabis products sold online or in dispensaries are often labeled inaccurately, with both under- and over-reporting of THC and CBD content.28-30 Some CBD products have been found to have almost no CBD at all.29,30 The unreliability of product labeling makes it difficult to predict the effects of CBD products that are not subject to FDA purity standards for medications or dietary supplements. It also raises questions about the sources of CBD and the reliability of dosing in the studies discussed above.
Why might CBD work as an antipsychotic? Although CBD has minimal affinity for cannabinoid receptors, it appears to act as a partial agonist of dopamine D2 receptors and an agonist at 5-HT1A receptors, with overall effects that decrease mesolimbic dopamine activity.31,32 In addition, CBD increases the availability of the endogenous cannabinoid anandamide, which may have antipsychotic properties.14,33
Now that the FDA has approved CBD manufactured by a pharmaceutical company, should it be prescribed “off-label” for patients with schizophrenia? This is the “million dollar question,” with insufficient evidence to provide a clear answer. It should now be possible to prescribe FDA-approved CBD for off-label purposes, including the treatment of schizophrenia and other psychiatric disorders. No doubt, some clinicians are already doing so. This will predictably yield more anecdotal evidence about efficacy and adverse effects in the future, but there is not yet adequate evidence to support an FDA indication for CBD in schizophrenia. Additional studies of CBD for schizophrenia are ongoing.
Bottom Line
Cannabidiol (CBD) is becoming increasingly popular based on its purported health benefits, but the evidence supporting a therapeutic role in psychiatry is preliminary at best. Although CBD is now available by prescription as an FDA-approved drug for the treatment of 2 rare forms of epilepsy, its benefits in patients with schizophrenia are uncertain based on mixed results in clinical trials.
Related Resources
- Clinicaltrials.gov. Studies of “cannabidiol” and “schizophrenia.” U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Schizophrenia&term=cannabidiol.
- Grinspoon P. Cannabidiol (CBD) – what we know and what we don’t. Harvard Health Blog. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-wedont-2018082414476. Published August 24, 2018.
Drug Brand Names
Cannabidiol • Epidiolex
Dronabinol • Marinol
Risperidone • Risperdal
Over the past few decades, it has become increasingly clear that cannabis use can increase the risk of developing a psychotic disorder and worsen the course of existing schizophrenia in a dose-dependent fashion.1-3 Beyond psychosis, although many patients with mental illness use cannabis for recreational purposes or as purported “self-medication,” currently available evidence suggests that marijuana is more likely to represent a harm than a benefit for psychiatric disorders4 (Box4-8). Our current state of knowledge therefore suggests that psychiatrists should caution their patients against using cannabis and prioritize interventions to reduce or discontinue use, especially among those with psychotic disorders.
Box
Data from California in 2006—a decade after the state’s legalization of “medical marijuana”—revealed that 23% of patients in a sample enrolled in medical marijuana clinics were receiving cannabis to treat a mental disorder.5 That was a striking statistic given the dearth of evidence to support a benefit of cannabis for psychiatric conditions at the time, leaving clinicians who provided the necessary recommendations to obtain medical marijuana largely unable to give informed consent about the risks and benefits, much less recommendations about specific products, routes of administration, or dosing. In 2019, we know considerably more about the interaction between cannabinoids and mental health, but research findings thus far warrant more caution than enthusiasm, with one recent review concluding that “whenever an association is observed between cannabis use and psychiatric disorders, the relationship is generally an adverse one.”4
Some critics have argued that the medical marijuana industry represents little more than a front for recreational use. In California and other states that have legalized recreational use, that claim has been rendered all but moot, although the public remains curious about the potential health benefits of cannabinoids and will likely continue to look to clinicians for advice. For those seeking guidance from evidence-based research, the existing state of knowledge can seem like a “Wild West” of anecdotal subjective reports, biased opinions, and uncontrolled clinical studies. Cannabis remains a Schedule I drug at the federal level, and quality clinical research has been limited to a relatively modest number of randomized controlled trials (RCTs), mostly involving FDA-approved cannabinoids rather than smoked cannabis. Randomized controlled trials that have involved smoked marijuana have generally involved low-potency delta-9-tetrahydrocannabinol (THC) cannabis that may not reflect the same therapeutic and adverse effects of the increasingly high potency cannabis now available on the street and in dispensaries.
In psychiatry, a few RCTs are underway exploring cannabis as a viable treatment for mental disorders (eg, posttraumatic stress disorder), but none have yet been completed or published. At best, retrospective studies to date have failed to support a consistent benefit of cannabis for any psychiatric disorder and at worst increasingly suggest a negative impact on psychotic, mood, and anxiety disorders.4,6 Meanwhile, synthetic cannabinoid receptor agonists (eg, “Spice” products) have come to represent a clear public health risk, with both medical and psychiatric toxicity.7
A more cautiously optimistic case for the therapeutic potential of cannabinoids in psychiatry could be made for cannabidiol (CBD), which may possess anxiolytic, antipsychotic, and neuroprotective properties.8 Based on its purported health benefits, it is possible that CBD may even gain widespread popularity as a food supplement. Because a pharmaceutically-manufactured form of CBD was recently FDA-approved for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, off-label prescribing of CBD for psychiatric disorders can be anticipated. While there is not yet sufficient evidence about risks and benefits to justify CBD being recommended broadly in psychiatry, that same informational vacuum has not stopped eager patients from seeking approval for cannabis, and some physicians from providing it.
Despite that conclusion, because cannabis is classified as a Schedule I drug by the US Drug Enforcement Agency, clinical research investigating the risks and benefits of cannabis has been limited. It therefore remains possible that cannabis, or individual cannabinoids such as cannabidiol (CBD), may yet find a therapeutic niche in psychiatry. This article reviews evidence on CBD for the treatment of schizophrenia.
Cannabinergic drugs as potential antipsychotics
Although the bulk of evidence indicates a harmful effect of cannabis in individuals with or at risk for psychosis, there have been a few published cases of schizophrenia improving with dronabinol, an FDA-approved, synthetic form of delta-9-tetrahydrocannabinol (THC).9,10 THC is the constituent of cannabis that produces euphoric effects. These provocative findings have not been replicated in controlled clinical trials, but suggest at least the theoretical possibility of idiosyncratic benefits from THC for some individuals within the psychotic spectrum.
Still, given that most available evidence supports that THC has a harmful effect on psychosis and psychosis risk, researchers have instead performed randomized controlled trials (RCTs) to investigate a possible therapeutic role for medications that oppose the agonist effects of THC at cannabinoid type 1 (CB1) receptors. To date, 2 RCTs comparing rimonabant, a CB1 inverse agonist, with placebo (PLB) in patients with schizophrenia have failed to demonstrate any benefit for psychotic symptoms or cognitive deficits.11,12 A third trial examining rimonabant for people diagnosed with schizophrenia who were overweight found significant benefits for anxiety and depressive symptoms, but none for positive symptoms or the primary outcome of weight loss.13 While these results are discouraging, the role of THC in precipitating psychosis suggests that novel agents opposing the actions of THC on the cannabinoid system could have antipsychotic properties.14
Cannabidiol: An antipsychotic medication?
In contrast to THC, CBD has minimal euphorigenic properties and has recently been heralded in the popular press as a “miracle drug” with benefits for medical and psychiatric disorders alike.15 It has even been speculated that it could become a popular food supplement.16 In 2018, the FDA gave full approval to a pharmaceutically manufactured form of CBD (brand name: Epidiolex) as a novel treatment for 2 rare and severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,17 based on RCTs supporting its efficacy for these often refractory and life-threatening conditions.18-20
In psychiatry, there have not yet been enough robust clinical studies to support broad therapeutic claims for CBD as a treatment for any mental disorder.21 However, there is growing evidence that CBD has potential as an antipsychotic medication. In 1995, the first case report was published describing the efficacy of CBD, 1,500 mg/d, as standalone therapy in a single individual with schizophrenia.22 In 2006, the same research group followed up with a case series in which only 1 out of 3 patients with treatment-refractory schizophrenia improved with flexible dosing of CBD to a maximum dose of 1,280 mg/d.23
There have been 3 published RCTs exploring the efficacy of CBD in schizophrenia (Table24-26). The first study, published in 2012, included 39 adults with schizophrenia who were randomized to 800 mg/d of CBD or amisulpride (AMS), a second-generation antipsychotic that is popular in Europe but is not available in the United States.24 Over 4 weeks of randomized treatment, CBD resulted in as much improvement in overall symptoms and positive symptoms as AMS, and improvement of negative symptoms was significantly greater with CBD. Compared with patients treated with antipsychotic medication, patients who were treated with CBD had fewer extrapyramidal symptoms, less weight gain, and less prolactin elevation. This initial trial suggests that CBD might be as efficacious in schizophrenia as antipsychotic medication, without its burdensome adverse effects. However, this is the only RCT of CBD monotherapy published to date.
Continue to: Two other recently published RCTs...
Two other recently published RCTs compared CBD with PLB as add-on therapy to antipsychotics. McGuire et al25 compared CBD, 1,000 mg/d, to PLB over 6 weeks in 88 patients with schizophrenia. Positive symptom improvement was statistically greater with CBD than with PLB, although the magnitude of clinical change was modest (using the Positive and Negative Syndrome Scale [PANSS] positive symptom subscale: −3.2 points for CBD vs −1.7 points for PLB). Changes in PANSS total score and subscales for general and negative symptoms were not significantly different between treatment groups. There was also no significant difference in overall change in neurocognitive symptoms, although post-hoc analysis revealed significantly greater improvement in motor speed for patients treated with CBD. More than twice the number of patients treated with CBD were rated as “much improved” by the Clinical Global Impressions scale compared with patients treated with PLB, but this was not a statistically significant finding, and most patients experienced only “minimal” or “no improvement.” In terms of adverse events, there were no significant differences between patients in the CBD and PLB groups. Although this study is technically “positive” for CBD and suggests minimal adverse effects, it is not clear whether the statistically significant positive symptom improvements (+1.5 PANSS points for CBD over PLB) were clinically significant.
The most recently published placebo-controlled RCT of CBD as add-on therapy to antipsychotic medication included 36 patients with schizophrenia treated over 6 weeks.26 In this study, there was no benefit of CBD, 600 mg/d, on any PANSS score outcome (total, general, positive, or negative symptoms). For the primary outcome of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, there were no significant drug × time effects, and post-hoc analyses showed that only patients treated with PLB improved with time. Sedation was more common among patients treated with CBD compared with PLB.
Making sense of the data
There have been mixed results from the few case reports and 3 RCTs of patients with schizophrenia who were treated with CBD. How can we resolve these disparate findings? A few possible interpretations of the data that warrant clarification through additional research include:
Dosing. In the first case report with positive results, CBD was dosed at 1,500 mg/d,22 whereas in the subsequent case series with mixed results, the maximum allowable dose of CBD was 1,280 mg/d.23 Likewise, in the RCTs, positive results were found when CBD was dosed at 800 to 1,000 mg/d,24,25 but not at 600 mg/d.26 The efficacy of CBD for schizophrenia might depend on higher doses.
Treatment resistance. In the second case series in which only 1 out of 3 patients responded to treatment with CBD,23 the patients had demonstrated previous nonresponse to at least 2 first-generation antipsychotics (FGAs) and risperidone, 6 mg/d. In the RCTs, all patients were antipsychotic-responsive.24-26 Cannabidiol may not be as effective for patients with treatment-refractory schizophrenia as it is for patients with schizophrenia who respond to antipsychotics.
Continue to: Clinical stability
Clinical stability. Within the RCTs, the greatest response was observed in the study that enrolled patients who were hospitalized with acute symptoms of schizophrenia.23 In the 2 studies that found either modest or no benefit with CBD, the patients had been stabilized on antipsychotic medications prior to randomization. Cannabidiol may offer limited benefit as add-on therapy to patients who have already responded to antipsychotic treatment, where there is “less room” for additional improvement.
Monotherapy. Both the case reports22,23 and the RCT with the most robust positive findings24 involved treatment with CBD as monotherapy. For some patients with schizophrenia, CBD might be effective as standalone therapy as an alternative to antipsychotics that is better tolerated. Adding CBD to antipsychotic therapy might be redundant and therefore less effective.
Answering questions about CBD
Cannabidiol is becoming increasingly popular for its purported health benefits. The mixed results of the few studies published on CBD for schizophrenia place clinicians in a difficult position when attempting to answer questions about how cannabinoids might fit into treatment of patients with psychosis. Consider the following:
Is cannabis helpful for patients with schizophrenia? No. Aside from the few case reports suggesting that FDA-approved THC (dronabinol) can improve symptoms in some patients,9,10 most of the evidence from anecdotal reports and both experimental and observational studies indicate that cannabis, THC, and synthetic cannabinoids have a harmful effect in patients with or at risk for psychosis.1-3
If you are considering recommending some form of cannabis to patients with schizophrenia, what kind should you recommend? Recommending or encouraging cannabis use for patients with psychosis is ill-advised. Although certain types of cannabis might contain more THC (eg, Cannabis indica vs Cannabis sativa) or variable amounts of CBD, in general the amount of CBD in whole leaf cannabis is minimal, with the ratio of THC to CBD increasingly significantly over the past decade.3,27 Most forms of cannabis should therefore be avoided by individuals with or at risk for psychotic disorders.
Continue to: What about CBD oil and other CBD products sold in dispensaries?
What about CBD oil and other CBD products sold in dispensaries? Cannabidiol is increasingly available in various forms based on its ability to be designated as a legal hemp product (containing <0.3% THC) at the federal level or as a cannabinoid in states where cannabis is legal. However, several studies have now shown that cannabis products sold online or in dispensaries are often labeled inaccurately, with both under- and over-reporting of THC and CBD content.28-30 Some CBD products have been found to have almost no CBD at all.29,30 The unreliability of product labeling makes it difficult to predict the effects of CBD products that are not subject to FDA purity standards for medications or dietary supplements. It also raises questions about the sources of CBD and the reliability of dosing in the studies discussed above.
Why might CBD work as an antipsychotic? Although CBD has minimal affinity for cannabinoid receptors, it appears to act as a partial agonist of dopamine D2 receptors and an agonist at 5-HT1A receptors, with overall effects that decrease mesolimbic dopamine activity.31,32 In addition, CBD increases the availability of the endogenous cannabinoid anandamide, which may have antipsychotic properties.14,33
Now that the FDA has approved CBD manufactured by a pharmaceutical company, should it be prescribed “off-label” for patients with schizophrenia? This is the “million dollar question,” with insufficient evidence to provide a clear answer. It should now be possible to prescribe FDA-approved CBD for off-label purposes, including the treatment of schizophrenia and other psychiatric disorders. No doubt, some clinicians are already doing so. This will predictably yield more anecdotal evidence about efficacy and adverse effects in the future, but there is not yet adequate evidence to support an FDA indication for CBD in schizophrenia. Additional studies of CBD for schizophrenia are ongoing.
Bottom Line
Cannabidiol (CBD) is becoming increasingly popular based on its purported health benefits, but the evidence supporting a therapeutic role in psychiatry is preliminary at best. Although CBD is now available by prescription as an FDA-approved drug for the treatment of 2 rare forms of epilepsy, its benefits in patients with schizophrenia are uncertain based on mixed results in clinical trials.
Related Resources
- Clinicaltrials.gov. Studies of “cannabidiol” and “schizophrenia.” U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Schizophrenia&term=cannabidiol.
- Grinspoon P. Cannabidiol (CBD) – what we know and what we don’t. Harvard Health Blog. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-wedont-2018082414476. Published August 24, 2018.
Drug Brand Names
Cannabidiol • Epidiolex
Dronabinol • Marinol
Risperidone • Risperdal
1. Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: what the evidence says. Current Psychiatry. 2011;10(9):49-58.
2. Radhakrishan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
3. Pierre JM. Risks of increasingly potent cannabis: joint effects of potency and frequency. Current Psychiatry. 2016;16(2):14-20.
4. Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-255.
5. Nunberg H, Kilmer B, Pacula RL, et al. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011;4(1):1.
6. Wilkinson ST, Radhakrishnan, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
7. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus. 2016;38(3):344-366.
8. Crippa JA, Guimarães FS, Campos A, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009.
9. Schwarz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
10. Schwarz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
11. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975-984.
12. Boggs DL, Kelly DL, McMahon RP, et al. Rimonabant for neurocognition in schizophrenia: a 16-week double blind placebo controlled trial. Schizophr Res. 2012;134(2-3):207-210.
13. Kelly DL, Gorelick DA, Conley RR, et al. Effects of cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: a randomized, double-blind, pilot study. J Clin Psychopharmacol. 2011;31(1):86-91.
14. Leweke FM, Mueller JK, Lange B, et al. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604-612.
15. Halperin A. What is CBD? The ‘miracle’ cannabis compound that doesn’t get you high. The Guardian. https://www.theguardian.com/society/2018/may/28/what-is-cbd-cannabidiol-cannabis-medical-uses. Published May 28, 2018. Accessed April 3, 2019.
16. Pierre J. Coca, cola, and cannabis: psychoactive drugs as beverages. Psychology Today (blog) Psych Unseen. https://www.psychologytoday.com/us/blog/psych-unseen/201810/coca-cola-and-cannabis-psychoactive-drugs-beverages. Published October 1, 2018. Accessed April 3, 2019.
17. U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Published June 25, 2018. Accessed April 3, 2019.
18. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
19. Thiele EA, March ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096.
20. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
21. Khoury JM, Neves MCLD, Rogue MAV, et al. Is there a role of cannabidiol in psychiatry? World J Biol Psychiatry. 2017:1-16.
22. Zuardi AW, Morais SL, Guimares FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
23. Zuardi AW, Hallak JEC, Dursun SM. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
24. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15.
25. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
26. Boggs DL, Surti I, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol. 2018;235(7):1923-1932.
27. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016; 79(7):613-619.
28. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2492.
29. Ruth AC, Gryniewicz-Ruzicka CM, Trehy ML, et al. Consistency of label claims of internet-purchased hemp oil and cannabis products as determined using IMS and LC-MS: a marketplace study. J Reg Sci. 2016;3:1-6.
30. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
31. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. doi: 10.1038/tp.2016.195.
32. Renard J, Norris C, Rushlow W, et al. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;157-165.
33. Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res. 2016;176(2-3):281-290.
1. Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: what the evidence says. Current Psychiatry. 2011;10(9):49-58.
2. Radhakrishan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
3. Pierre JM. Risks of increasingly potent cannabis: joint effects of potency and frequency. Current Psychiatry. 2016;16(2):14-20.
4. Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-255.
5. Nunberg H, Kilmer B, Pacula RL, et al. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011;4(1):1.
6. Wilkinson ST, Radhakrishnan, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
7. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus. 2016;38(3):344-366.
8. Crippa JA, Guimarães FS, Campos A, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009.
9. Schwarz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
10. Schwarz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
11. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975-984.
12. Boggs DL, Kelly DL, McMahon RP, et al. Rimonabant for neurocognition in schizophrenia: a 16-week double blind placebo controlled trial. Schizophr Res. 2012;134(2-3):207-210.
13. Kelly DL, Gorelick DA, Conley RR, et al. Effects of cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: a randomized, double-blind, pilot study. J Clin Psychopharmacol. 2011;31(1):86-91.
14. Leweke FM, Mueller JK, Lange B, et al. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604-612.
15. Halperin A. What is CBD? The ‘miracle’ cannabis compound that doesn’t get you high. The Guardian. https://www.theguardian.com/society/2018/may/28/what-is-cbd-cannabidiol-cannabis-medical-uses. Published May 28, 2018. Accessed April 3, 2019.
16. Pierre J. Coca, cola, and cannabis: psychoactive drugs as beverages. Psychology Today (blog) Psych Unseen. https://www.psychologytoday.com/us/blog/psych-unseen/201810/coca-cola-and-cannabis-psychoactive-drugs-beverages. Published October 1, 2018. Accessed April 3, 2019.
17. U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Published June 25, 2018. Accessed April 3, 2019.
18. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
19. Thiele EA, March ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096.
20. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
21. Khoury JM, Neves MCLD, Rogue MAV, et al. Is there a role of cannabidiol in psychiatry? World J Biol Psychiatry. 2017:1-16.
22. Zuardi AW, Morais SL, Guimares FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
23. Zuardi AW, Hallak JEC, Dursun SM. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
24. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15.
25. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
26. Boggs DL, Surti I, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol. 2018;235(7):1923-1932.
27. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016; 79(7):613-619.
28. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2492.
29. Ruth AC, Gryniewicz-Ruzicka CM, Trehy ML, et al. Consistency of label claims of internet-purchased hemp oil and cannabis products as determined using IMS and LC-MS: a marketplace study. J Reg Sci. 2016;3:1-6.
30. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
31. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. doi: 10.1038/tp.2016.195.
32. Renard J, Norris C, Rushlow W, et al. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;157-165.
33. Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res. 2016;176(2-3):281-290.