User login
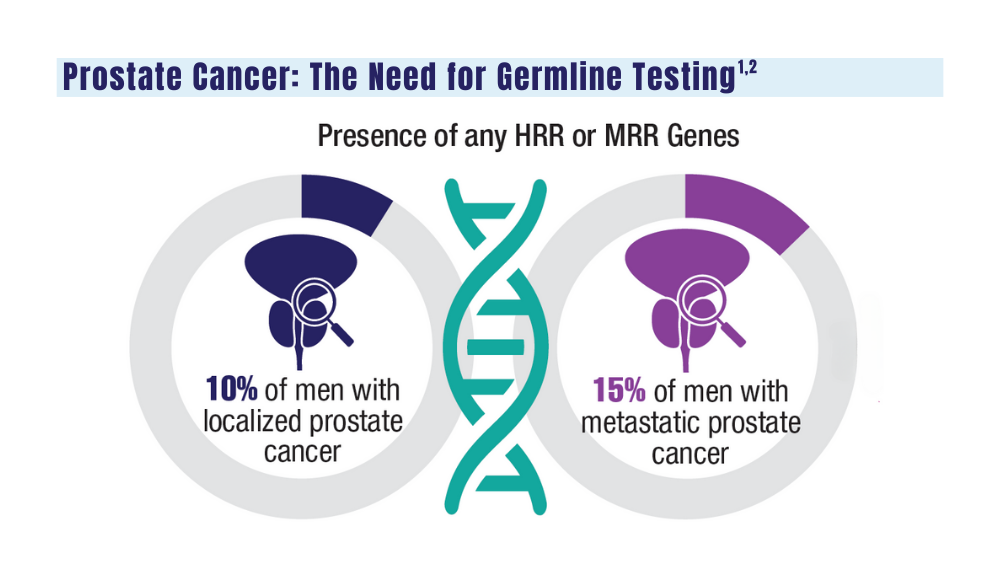
1. Sokolova A, Cheng H. Germline testing in prostate cancer: when and who to test. Oncology (Williston Park). 2021;35(10):645-653. doi:10.46883/ONC.2021.3510.0645
2. Tuffaha H, Edmunds K, Fairbairn D, et al. Guidelines for genetic testing in prostate cancer: a scoping review. Prostate Cancer Prostatic Dis. 2023 May 18. doi:10.1038/s41391-023-00676-0
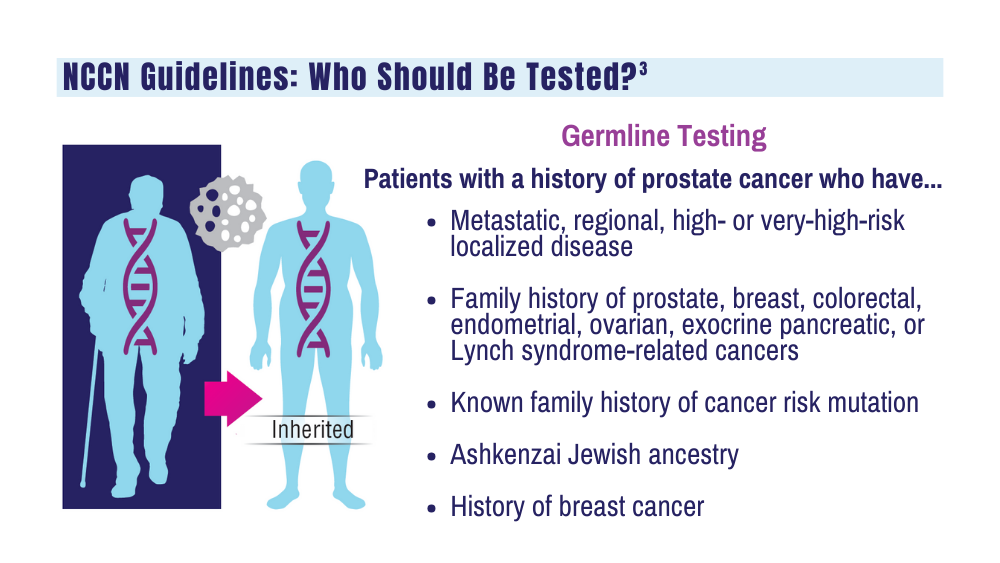
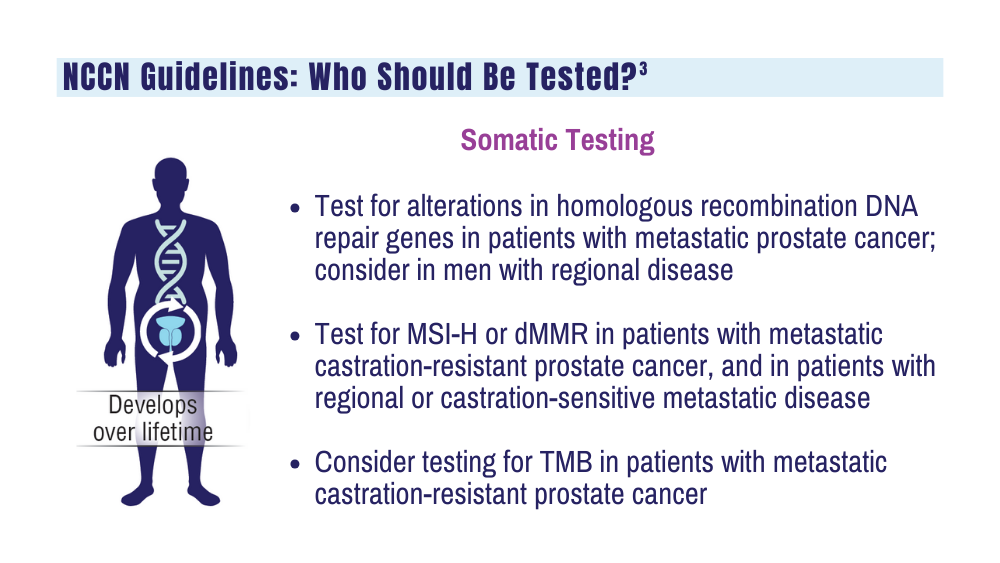
3. National Comprehensive Cancer Network. NCCN clinical practice guidelines for prostate cancer. Version 4.2023. September 7, 2023. Accessed December 20, 2023. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
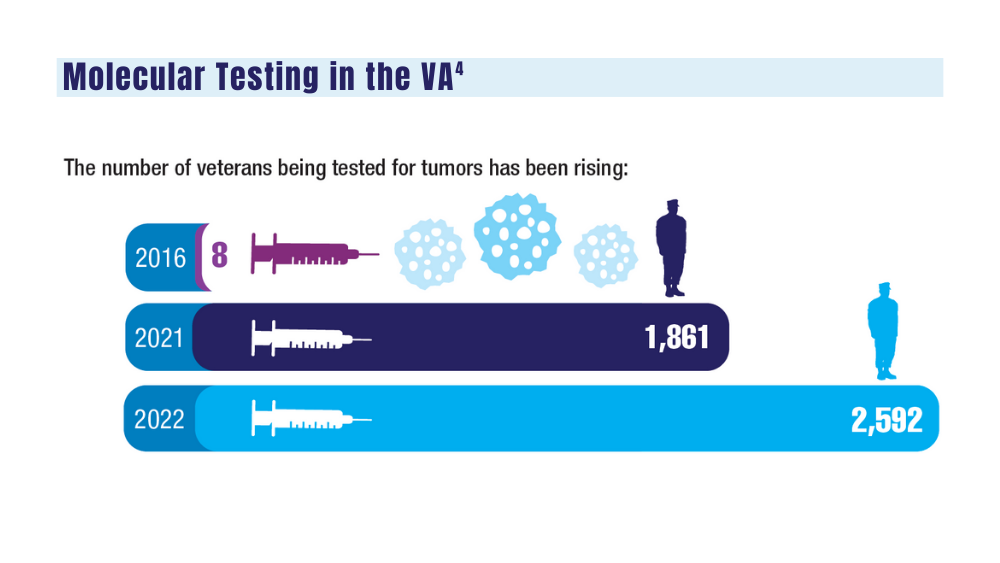
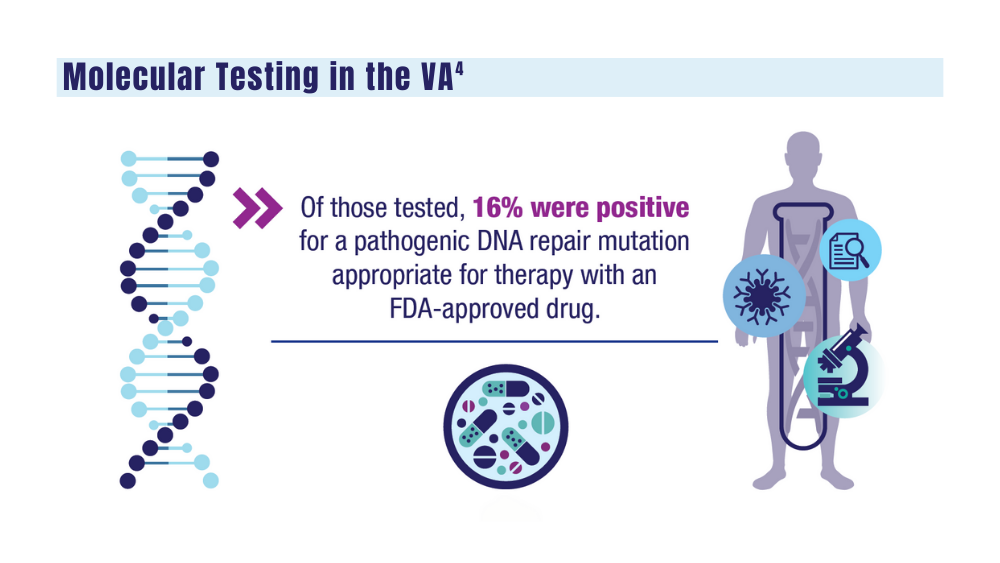
4. National Precision Oncology Program. PMID 26149669 (e-mail, December 13, 2023).
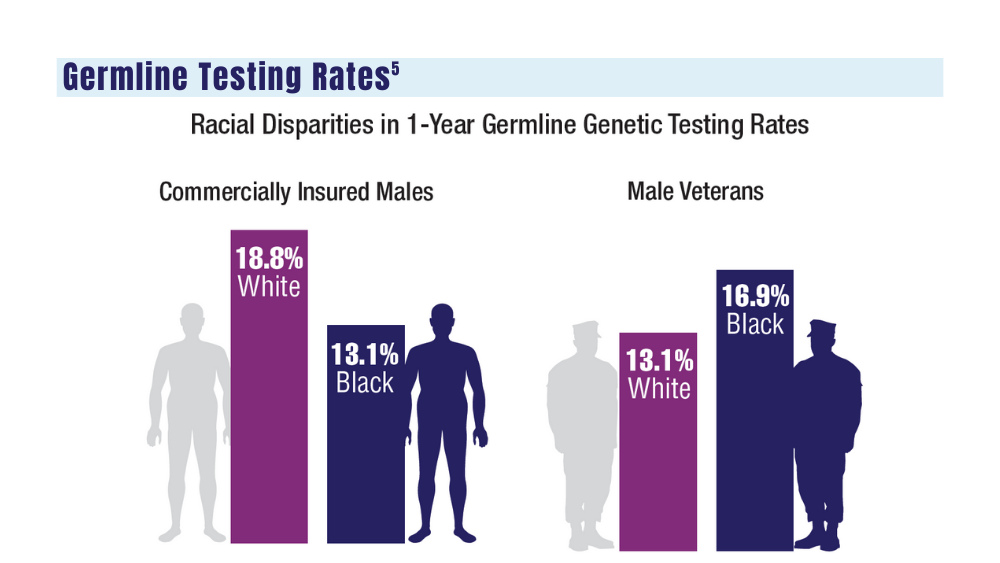
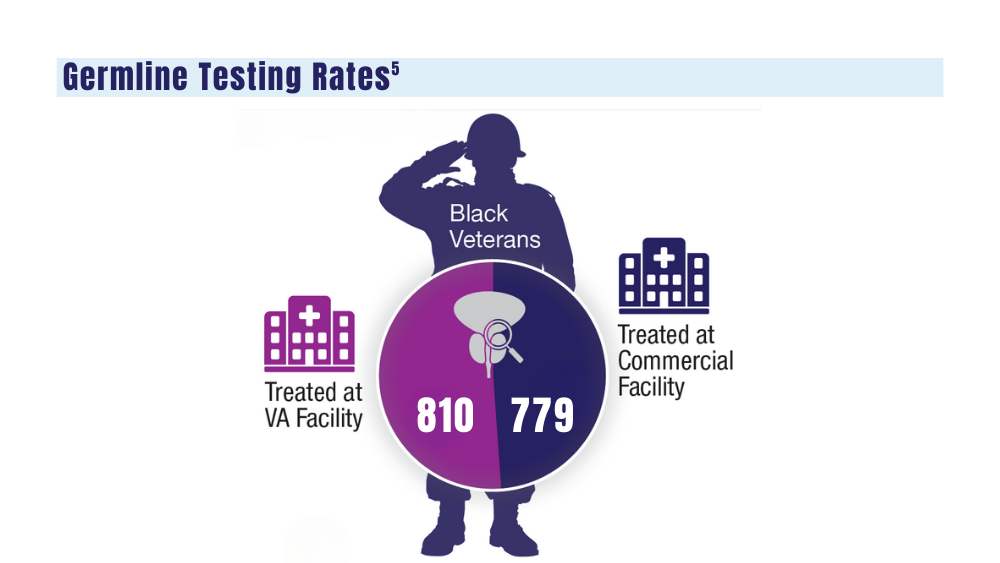
5. Shevach J, Lynch J, Candelieri-Surette D, et al. Racial disparities in germline testing among men with pancreas, breast and metastatic prostate cancers in two health systems. J Clin Oncol. 2023;41(16 suppl):abstract 10549. https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.16_suppl.10549
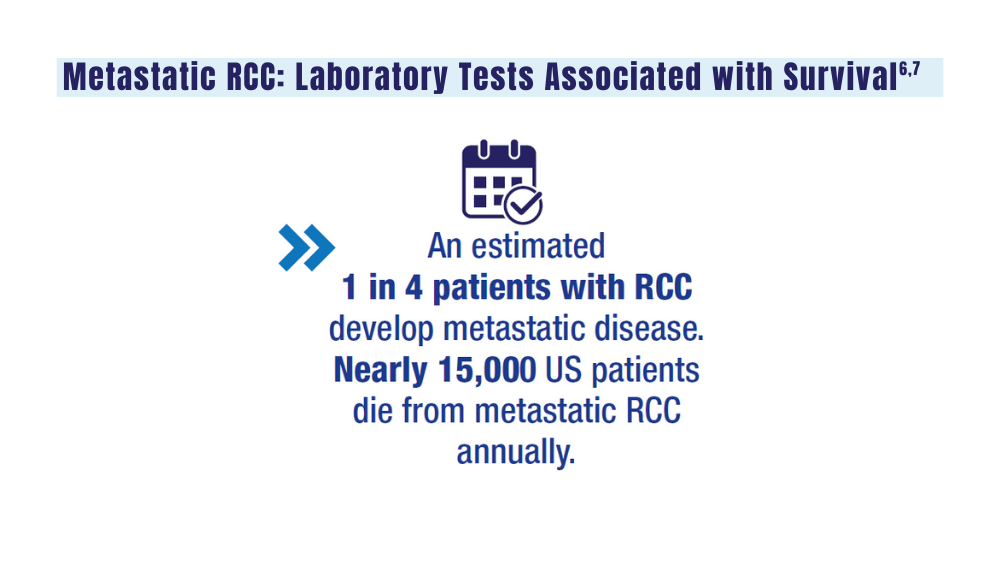
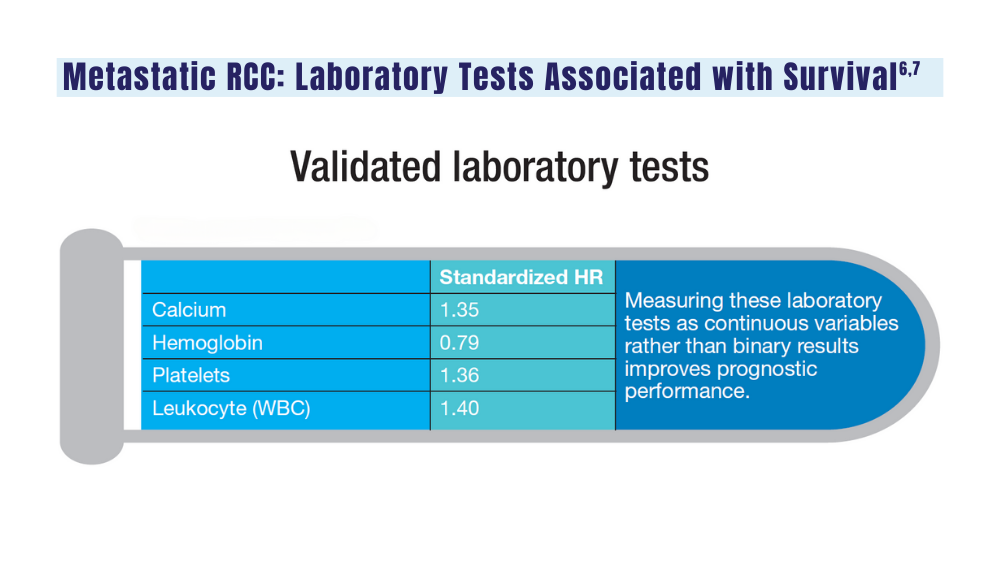
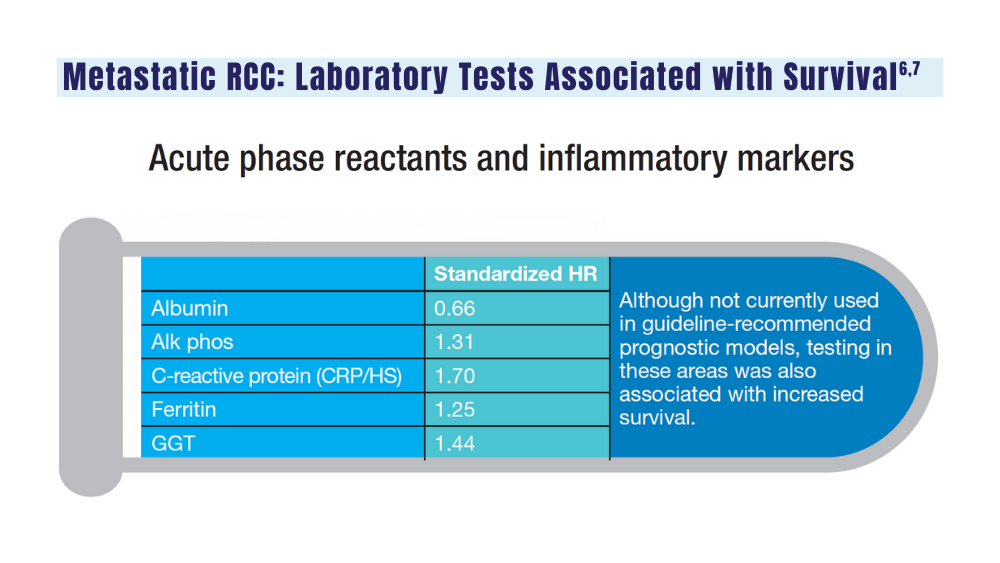
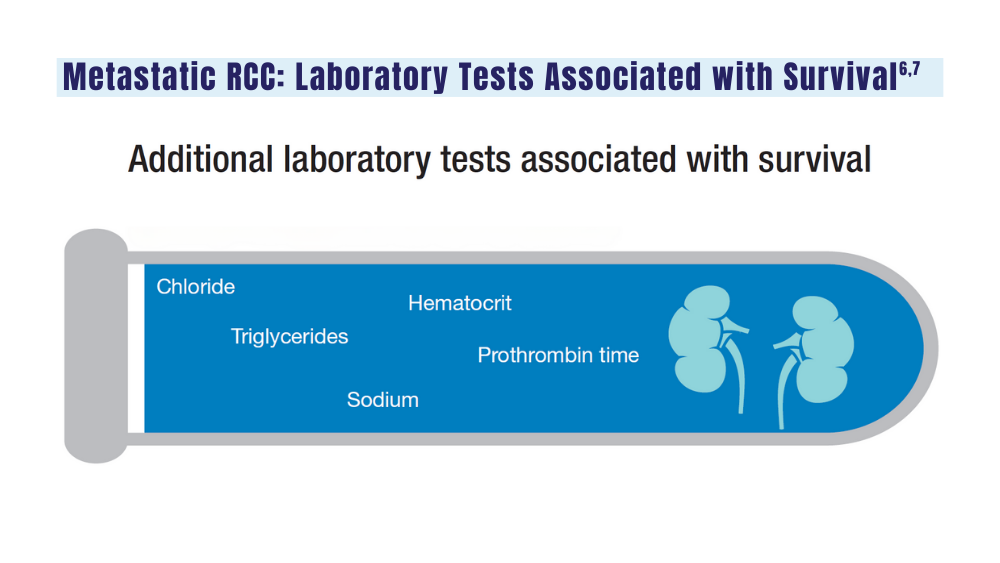
6. Velaer K, Thomas IC, Yang J, et al. Clinical laboratory tests associated with survival in patients with metastatic renal cell carcinoma: a laboratory wide association study (LWAS). Urol Oncol. 2022;40(1):12.e23-12.e30. doi:10.1016/j.urolonc.2021.08.011
7. Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141-148. doi:10.1016/S1470-2045(12)70559-4
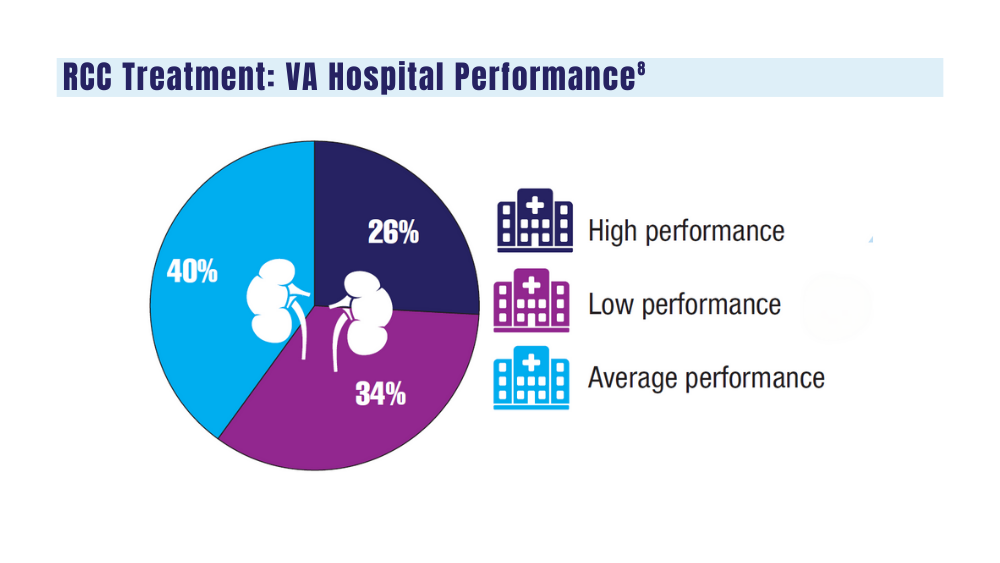
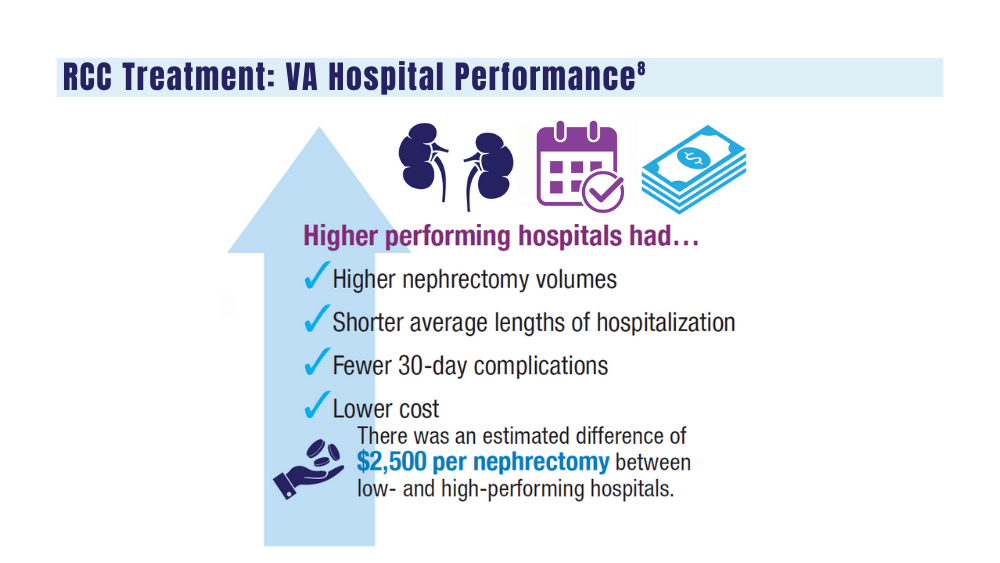
8. Aguilar Palacios D, Wilson B, Michael P, et al. A novel metric for hospital quality in kidney cancer surgery: a Veterans Affairs National Health System validation of concept. Urol Pract. 2022;9(3):237-245. doi:10.1097/UPJ.0000000000000294
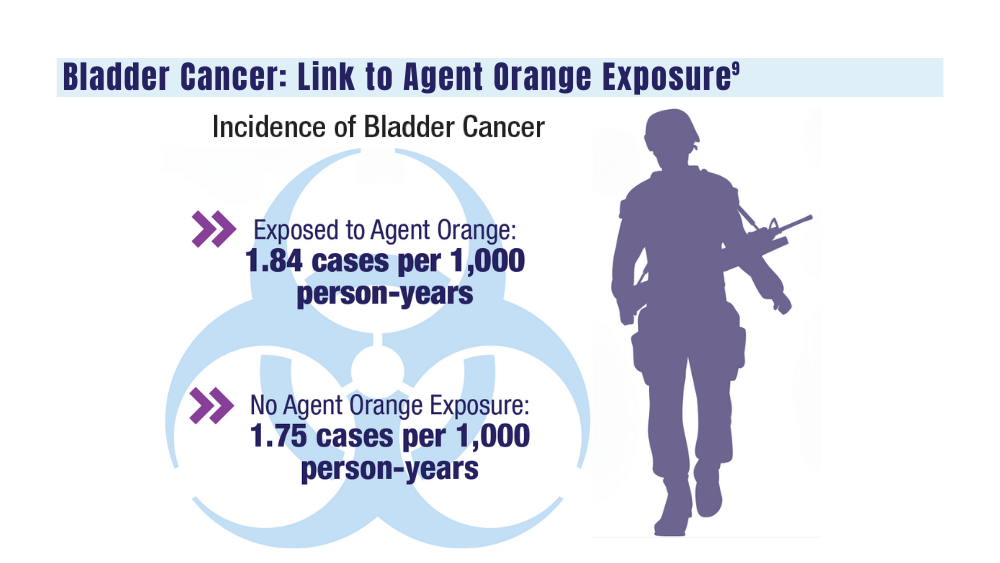
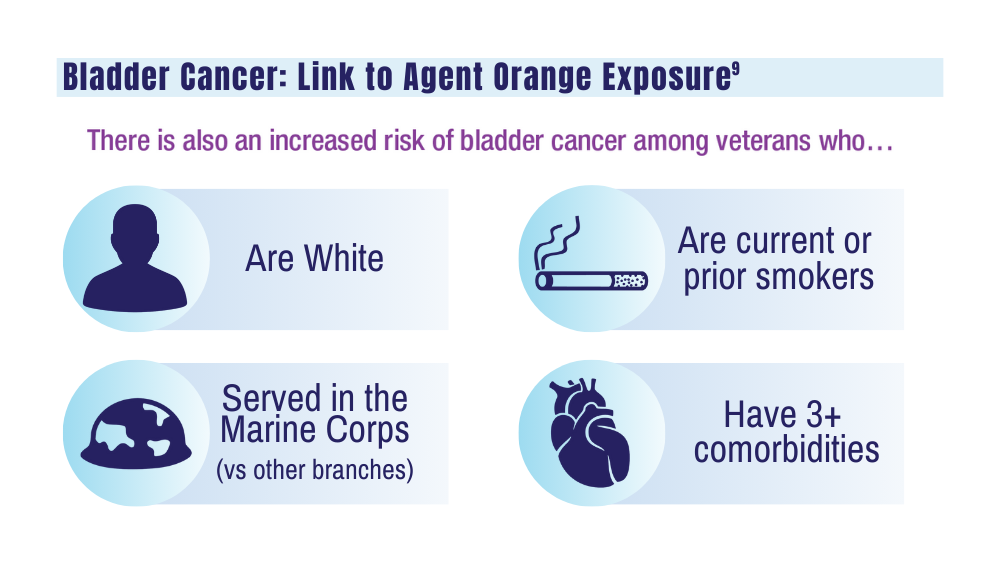
9. Williams SB, Janes JL, Howard LE, et al. Exposure to Agent Orange and risk of bladder cancer among US veterans. JAMA Netw Open. 2023;6(6):e2320593. doi:10.1001/jamanetworkopen.2023.20593
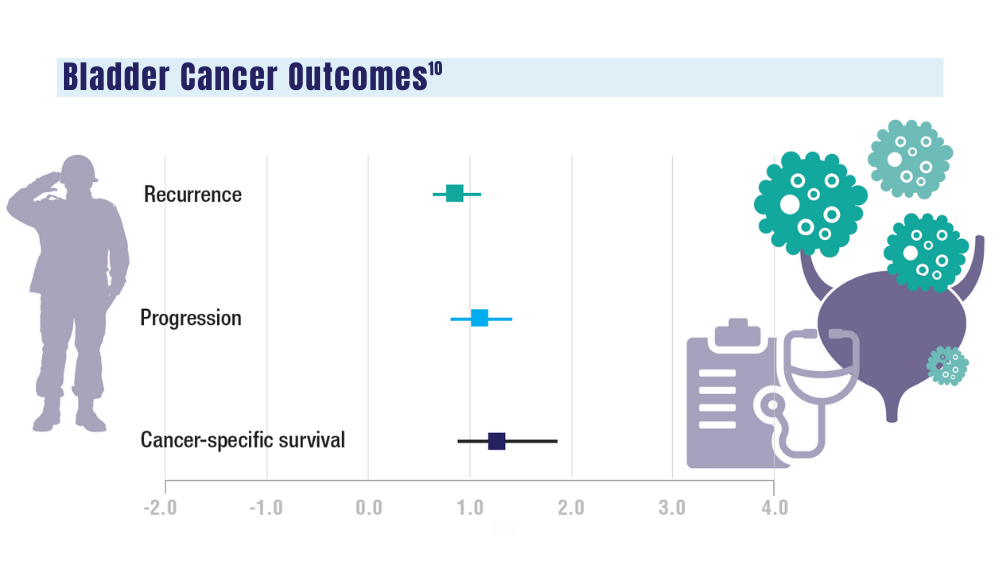
10. Penn T, Borza T, Liou JI, et al. Impact of Agent Orange exposure on non-muscle invasive bladder cancer outcomes. Urology. 2023;182:175-180. doi:10.1016/j.urology.2023.08.037
1. Sokolova A, Cheng H. Germline testing in prostate cancer: when and who to test. Oncology (Williston Park). 2021;35(10):645-653. doi:10.46883/ONC.2021.3510.0645
2. Tuffaha H, Edmunds K, Fairbairn D, et al. Guidelines for genetic testing in prostate cancer: a scoping review. Prostate Cancer Prostatic Dis. 2023 May 18. doi:10.1038/s41391-023-00676-0
3. National Comprehensive Cancer Network. NCCN clinical practice guidelines for prostate cancer. Version 4.2023. September 7, 2023. Accessed December 20, 2023. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
4. National Precision Oncology Program. PMID 26149669 (e-mail, December 13, 2023).
5. Shevach J, Lynch J, Candelieri-Surette D, et al. Racial disparities in germline testing among men with pancreas, breast and metastatic prostate cancers in two health systems. J Clin Oncol. 2023;41(16 suppl):abstract 10549. https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.16_suppl.10549
6. Velaer K, Thomas IC, Yang J, et al. Clinical laboratory tests associated with survival in patients with metastatic renal cell carcinoma: a laboratory wide association study (LWAS). Urol Oncol. 2022;40(1):12.e23-12.e30. doi:10.1016/j.urolonc.2021.08.011
7. Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141-148. doi:10.1016/S1470-2045(12)70559-4
8. Aguilar Palacios D, Wilson B, Michael P, et al. A novel metric for hospital quality in kidney cancer surgery: a Veterans Affairs National Health System validation of concept. Urol Pract. 2022;9(3):237-245. doi:10.1097/UPJ.0000000000000294
9. Williams SB, Janes JL, Howard LE, et al. Exposure to Agent Orange and risk of bladder cancer among US veterans. JAMA Netw Open. 2023;6(6):e2320593. doi:10.1001/jamanetworkopen.2023.20593
10. Penn T, Borza T, Liou JI, et al. Impact of Agent Orange exposure on non-muscle invasive bladder cancer outcomes. Urology. 2023;182:175-180. doi:10.1016/j.urology.2023.08.037
1. Sokolova A, Cheng H. Germline testing in prostate cancer: when and who to test. Oncology (Williston Park). 2021;35(10):645-653. doi:10.46883/ONC.2021.3510.0645
2. Tuffaha H, Edmunds K, Fairbairn D, et al. Guidelines for genetic testing in prostate cancer: a scoping review. Prostate Cancer Prostatic Dis. 2023 May 18. doi:10.1038/s41391-023-00676-0
3. National Comprehensive Cancer Network. NCCN clinical practice guidelines for prostate cancer. Version 4.2023. September 7, 2023. Accessed December 20, 2023. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
4. National Precision Oncology Program. PMID 26149669 (e-mail, December 13, 2023).
5. Shevach J, Lynch J, Candelieri-Surette D, et al. Racial disparities in germline testing among men with pancreas, breast and metastatic prostate cancers in two health systems. J Clin Oncol. 2023;41(16 suppl):abstract 10549. https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.16_suppl.10549
6. Velaer K, Thomas IC, Yang J, et al. Clinical laboratory tests associated with survival in patients with metastatic renal cell carcinoma: a laboratory wide association study (LWAS). Urol Oncol. 2022;40(1):12.e23-12.e30. doi:10.1016/j.urolonc.2021.08.011
7. Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141-148. doi:10.1016/S1470-2045(12)70559-4
8. Aguilar Palacios D, Wilson B, Michael P, et al. A novel metric for hospital quality in kidney cancer surgery: a Veterans Affairs National Health System validation of concept. Urol Pract. 2022;9(3):237-245. doi:10.1097/UPJ.0000000000000294
9. Williams SB, Janes JL, Howard LE, et al. Exposure to Agent Orange and risk of bladder cancer among US veterans. JAMA Netw Open. 2023;6(6):e2320593. doi:10.1001/jamanetworkopen.2023.20593
10. Penn T, Borza T, Liou JI, et al. Impact of Agent Orange exposure on non-muscle invasive bladder cancer outcomes. Urology. 2023;182:175-180. doi:10.1016/j.urology.2023.08.037