User login
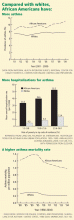
The last several decades have seen a dramatic surge in the prevalence of asthma. In 2009, there were an estimated 17.5 million adults and almost 7.1 million children with asthma in the United States,1 up from 9.5 million adults and slightly more than 5 million children in 1995.2
Multiple factors contribute to these disparities, including genetics, socioeconomic factors, cultural factors, health maintenance behaviors, provider-patient communication, air quality, and obesity.
GENETICS: 70% OF DESTINY?
The trend towards personalized medicine has spurred extensive research into the genetics of asthma. Studies in twins and familial aggregation studies suggest genetics plays a significant role, with estimates of the heritability of asthma as high as 70%.4,5 More than 100 candidate genes have been shown to be associated with asthma and atopy, 30 of them in five or more independent studies.6
Researchers face many challenges when investigating the genetics involved in asthma for a particular race. Race is both a biologic and a social construct and, as such, is a poor substitute for genetics. Race constitutes not only genetic differences in individuals, but also the behaviors, beliefs, and experiences that vary among races.
The clinical disease—the phenotype—is the product of the interaction of genes and these differing behaviors and exposures. Genetics can affect how environmental factors found in association with socioeconomic factors relate to asthma morbidity and mortality.
For example, as we will discuss below, African Americans are more likely than whites to be sensitized to cockroach allergen, even after controlling for socioeconomic variables that may be associated with greater exposure.7 High-level exposure to cockroach allergen in sensitized children has been associated with poor asthma outcomes.8 This suggests that a genetic difference may exist between African Americans and whites with respect to the potential to develop cockroach sensitization, and this difference may be of particular importance for those African Americans living in areas with higher levels of cockroach exposure.
Two polymorphisms
Two polymorphisms have garnered attention for their influence on African Americans with asthma:
TheADRB2gene. This gene codes for the beta-2 adrenergic receptor and resides at chromosome 5q13.9 The receptor is found on several types of cells in the lung, including airway smooth muscle and epithelial cells, and is responsible for the salutary effects of inhaled beta-2 agonists such as albuterol (eg, Proventil).
Allelic polymorphisms of this gene are clinically relevant. The substitution of arginine (Arg) for glycine (Gly) at codon 16 of this gene is responsible for differences in response to short-acting beta-2 agonists. The allelic frequency of Arg16 is lower in white Americans (39.3%) than in African Americans (49.2%), and thus African Americans are more likely to be homozygous for Arg16 (ie, to have the Arg/Arg genotype).10
People who are homozygous for Arg16 who use albuterol on a regular basis are at higher risk of untoward asthma outcomes.11 This is important, for several reasons. In general, adherence to inhaled corticosteroids is poor (not only in African Americans),12 and patients who do not take their inhaled corticosteroids as they should may rely on short-acting beta-2 agonists more frequently. Furthermore, African Americans may have a poorer response to the repeated doses of albuterol that are typically given in the emergency department and in the hospital for severe asthma exacerbations.13 Additionally, data suggest that Arg/Arg individuals have more frequent exacerbations independent of beta-agonist use,14 although curiously, patients who are homozygous for Arg16 have a greater benefit from single doses of short-acting beta-2 agonists than those who are Gly16 homozygous.15
TheCD14gene. An interesting relationship between innate immunity and asthma has recently been described. Polymorphisms of CD14, which codes for a receptor for endotoxin, have been uncovered. The single-nucleotide polymorphism variant thymine (T) at position −260 has been found in greater frequency in whites than in African Americans, who are more likely to have the cytosine (C) allele.16 An association between the CC genotype and atopy has been reported,16 although this has not been consistent.17
A possible explanation for these inconsistencies may lie in complex gene-environment interactions. The amount of endotoxin exposure may play a role in phenotypic expression. Individuals with the CC genotype were at lower risk of developing atopy when exposed to high levels of endotoxin; however, when exposed to lower levels of endotoxin, the CC genotype was associated with a higher risk of atopy.18 Nonfarm homes in westernized countries tend to have lower levels of endotoxin than farm homes, even in low-income urban areas.19 This implies that individuals with the CC allele, who are more likely to be African American, would be at greater risk for atopy in the United States. Greater knowledge of these types of gene-environment interactions may lead to improved understanding of the observations that have generated controversy concerning the “hygiene hypothesis.”
The details of how microbial exposure can influence the human immune response to antigen exposure are still being elucidated.20
These examples highlight not only the importance of genetics in the development of asthma, but also the role genes play in variation of treatment response and subsequent risk of morbidity and death. An understanding of these genetic differences among patients is clearly important for moving towards personalized treatment strategies for asthma.
Ancestry-informative markers
A developing strategy to assess the differences in asthma prevalence, severity, and response to treatment between racial groups is the use of ancestry-informative markers (AIMs).
AIMs are single-nucleotide polymorphisms that occur in varying allelic frequencies between ancestral groups, eg, continental Africans or European whites.21 AIMs provide an estimate of an individual’s proportion of ancestry—ie, of how “African” an African American is genetically.
African ancestry, determined using AIMs, was found to be associated with asthma in people living on the Caribbean coast of Colombia.22 However, one study found that AIMs could not predict an individual’s response to inhaled corticosteroids.23
Further research is necessary to find a technique to determine how groups of individuals can be characterized more precisely and managed more appropriately.
SOCIOECONOMIC FACTORS
African Americans living in low-income urban areas have an even greater prevalence of asthma and a greater risk of asthma-related morbidity and death than African Americans overall.3,24,25 Urban areas typically have a high proportion of residents living at or below the poverty level, and minorities often constitute a substantial proportion of the population in these areas. Evidence suggests that both African American race and lower socioeconomic status are independent risk factors for asthma prevalence, morbidity, and death.3,25
To provide better care for African Americans living in low-income urban areas, it is important to understand the factors that may be contributing to the higher morbidity and mortality rates in low-income urban areas.
Inadequate follow-up
Proper and routine follow-up for evaluation of asthma symptoms is essential for appropriate management. The Expert Panel Report 3 (EPR-3) of the National Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma,26 published in 2007, recommends that patients be seen at least every 6 months if they have been experiencing good control. While gaining control, patients should be seen every 2 to 6 weeks.26
Despite these recommendations, numerous studies have suggested that African Americans do not receive adequate follow-up. Children who are poor, are nonwhite or Hispanic, or are underinsured are more likely to lack routine health care27 and, more specifically, routine asthma care.28 Low-income patients are also more likely to receive care in a large hospital-run clinic or neighborhood clinic,27,28 where continuity of care may be less than ideal.29 Even among patients enrolled in Medicaid or Medicare, African American children with a primary care provider have fewer asthma visits compared with white Medicaid-insured children.30
Insufficient follow-up care contributes to greater asthma morbidity, resulting in, for example, more emergency department visits for asthma in African Americans.27,31,32
Suboptimal care
Data also suggest that the quality of care that residents of low-income urban areas receive is often suboptimal. Many people living in low-income urban areas are not provided with the knowledge and tools to treat asthma exacerbations at home.33 African Americans are also less likely to be seen by an asthma specialist31,34 as recommended for those with moderate or severe asthma.26
The EPR-3 guidelines also stress the importance of inhaled corticosteroids as the preferred therapy for all patients with persistent asthma. Even after controlling for the number of primary care visits, insurance status, and disease severity, African Americans are less likely to receive a prescription for inhaled corticosteroids, or they receive the same dosage of inhaled corticosteroids in the face of more severe disease.31,33,35,36
The reasons for these differences in treatment are not fully understood but are likely multiple. Lack of access to an asthma specialist and financial or formulary constraints are some of the potential barriers to optimal asthma care outcomes.
Misdiagnosis in the acute setting may also be a source of less-than-ideal care, as patients seen in emergency departments may be misdiagnosed with viral infection or bronchitis.
African Americans may report different symptoms than whites
Intriguing studies suggest that African Americans report different symptoms while describing asthma exacerbations.
In one study, compared with whites, African Americans were less likely to report nocturnal symptoms, dyspnea, or chest pain during exacerbations.37 In another study, when given a methacholine challenge that induced a significant drop in forced expiratory volume in 1 second (FEV1), African Americans with asthma were more likely to complain of upper airway symptoms as opposed to lower airway symptoms, compared with white patients.38
The symptoms that African Americans describe, such as having a tight throat or voice, are not typically regarded as related to asthma; for this reason, such descriptions may be an obstacle to correct diagnosis, management, and follow-up.
Asthma care providers should be aware of these observations to ensure that their patients are managed appropriately.
Lack of social support
Living in a low-income urban area presents many challenges that can interfere with proper asthma control.
Asthma diagnosis, management, and morbidity are affected by family support.39 Patients with asthma who lack sufficient financial support for treatment, who lack adequate psychological support, and who have more major life stressors are at higher risk of untoward outcomes. Disruption and dysfunction of the family and the supports available have been associated with greater asthma morbidity.40–42 Unfortunately, these types of stressors are all too common in families living in low-income urban areas.43–45
Multiple stressors that can occur more often in low-income urban areas, including exposure to violent crime, have also been linked to greater asthma morbidity.45–47
POOR PHYSICIAN-PATIENT COMMUNICATION
A consistent theme in focus groups of African Americans living in inner-city areas is the perception that health care providers are not effectively communicating and taking the time to listen to their concerns.48,49 Respondents believed they had better insight into their illness than their providers, and for this reason were better able to manage their disease.
The importance of an optimal provider-patient relationship was highlighted by a prospective cohort study in which Medicaid children receiving care at physician’s offices with the highest cultural competency scores were more adherent with their asthma controller medications.50
MEDICATION ADHERENCE RATES ARE DISTURBINGLY LOW
Rates of medication adherence for chronic diseases is disturbingly low, and may be even worse for pulmonary diseases.51 Reported rates of adherence to asthma medications among all patients range from 50% to 60%.52,53 Several studies showed that African Americans have a lower rate of adherence than do whites,53–55 even after adjusting for multiple socioeconomic variables.56
Many explanations have been proposed for this discrepancy, and all likely play a role in particular environments. For example, the incidence of crime in the surrounding area was inversely related to medication adherence after adjusting for socioeconomic factors.57 African Americans may have more concern about side effects associated with inhaled corticosteroid use and may be less likely to understand how these drugs work.52,53 A poor provider-patient relationship has also been cited as a barrier to adherence.55,57 Finally, physicians are more likely to underestimate asthma severity in an African American patient than in a white patient.58
Taking the time to ensure that patients truly understand all aspects of their disease and establishing a health care environment that is culturally appropriate may have a significant impact in patients with asthma.
ENVIRONMENTAL EXPOSURES
Air quality contributes to the greater asthma morbidity observed in urban residents, including African Americans. While poor outdoor air quality has not been clearly linked to a higher incidence of asthma, it has been associated with greater asthma morbidity. Poor air quality may affect individuals of all races, but with respect to ambient pollutants such as particulate matter and diesel exhaust, outdoor air quality is worse in urban environments where greater proportions of people of low socioeconomic status reside.59,60
The most extensively studied components of air pollution are ozone, sulfur dioxide, and particulate matter. These pollutants have been associated with a higher rate of emergency department visits,61,62 worse asthma symptoms,63,64 and higher exhaled nitric oxide levels.65
Tobacco smoke
Despite the substantial success of smoking cessation efforts nationwide, exposure to tobacco smoke continues to be common and is a significant risk factor for poor asthma control. Recent data suggest that African Americans and whites have a similar prevalence of smoking,66 but a study found a very high prevalence in low-income African Americans.67
Active smoking has been associated with worse asthma control and a higher risk of death.68 People with asthma who smoke are less likely to improve in their lung function and symptom scores when treated with short courses of oral glucocorticoids compared with both nonsmokers and former smokers.69
Secondhand smoke hurts too. Many children living in low-income urban areas are exposed to secondhand smoke or environmental tobacco smoke.70,71 Passive exposure in children has been associated with worse asthma outcomes, and data suggest such exposure may be a cause of asthma.68,72–74
Environmental tobacco smoke has also been implicated in gene-environment interactions. Patients who are either homozygous or heterozygous for the Arg allele at codon 16 of the ADRB2 gene (discussed above) had significantly lower FEV1 and forced vital capacity (FVC) values when exposed to passive tobacco smoke. This difference was not seen in people who were not exposed.75
Cockroach allergen
The type and condition of a person’s housing also plays a role in asthma-related morbidity and death. Across several socioeconomic levels, it has been suggested that African Americans have poorer-quality housing compared with whites.76 Some of the conditions found in low-quality houses, such as interruptions in heat, plumbing leaks, and the presence of rodents, have been associated with a higher prevalence of asthma in the household.77
Cockroach allergen exposure and sensitization is a major contributor to asthma morbidity in African Americans, particularly those living in poorer urban areas where cockroach allergen may be the most common indoor allergen.8 Living in older housing in urban areas is associated with higher exposure to cockroach allergen, and with subsequent sensitization.78,79 Exposure to high levels of the major cockroach allergen, Bla g 1, in sensitized individuals has been linked to a greater risk of hospitalization and unscheduled medical visits for asthma. This was not found to be the case for other common indoor allergens, such as dust mite and cat dander.8
However, it is not only exposure to high cockroach allergen levels that puts African Americans at risk. African Americans living in low-income urban areas may also be more likely than whites living in low-income urban areas to become sensitized to cockroach allergen.7,80 This suggests a gene-environment interaction that may be unique to African Americans. Moreover, cockroach sensitization may occur early in life.81,82
While successful cockroach avoidance measures and environmental control may be challenging, such measures have been shown to decrease rates of asthma morbidity.83
OBESITY
Obesity has been linked to an ever-growing list of diseases, one of which is asthma. Obesity is not a unique challenge for African Americans, but recent data from the US Centers for Disease Control and Prevention show that African Americans have a 51% higher prevalence of obesity compared with whites.84
Obesity is a risk factor for greater asthma morbidity and is a significant challenge in the African American community. The rise in obesity rates has paralleled the rise in asthma in recent decades. The higher one’s body mass index, the higher one’s risk of asthma.85 This association appears to be stronger in people without concurrent atopic disease.86 Obesity has also been associated with a poorer response to inhaled corticosteroids and a higher risk of asthma exacerbations.87 Interestingly, significant weight loss has been associated with improvements in both asthma control and lung function.88,89
What is the mechanism?
The underlying pathogenic mechanisms have not been completely elucidated, and they are likely multiple.
Adipokines (cytokines secreted by adipocytes) have been implicated. Two of the most extensively studied adipokines are leptin and adiponectin. Leptin production is increased in obesity, and it has inflammatory effects on both the innate and adaptive immune systems.90 The opposite is true for adiponectin, which may have anti-inflammatory properties and which decreases as the body mass index increases.90 The precise role these molecules may have in lung disease is undergoing further investigation.
Mechanical alterations in lung function may also contribute. Obese people have a lower functional residual capacity and expiratory reserve volume. Breathing with a lower-volume functional residual capacity results in decreased airway diameter and contributes to increased airway resistance.90 The decreased airway diameter may alter the contractile properties of airway smooth muscle and lead to increased airway responsiveness.90 These differences are in addition to the lower mean values of common spirometry indices such as the FEV1 and FVC, found in nonasthmatic African Americans compared with whites.91
Data suggest these differences are primarily due to anthropometric factors, with nutritional and environmental factors playing a less significant role.92 On this basis, the American Thoracic Society recommends applying race-specific reference standards for use with spirometry in order to accurately gauge lung function in African Americans.
APPROPRIATE CARE AND EDUCATION
The cause of greater asthma prevalence and severity among African Americans is multifactorial. It is likely that a number of factors work together, rather than separately, in influencing the development of asthma and its course.
Some risk factors are avoidable, and it is important to identify and ameliorate them. Others are not preventable, but knowledge of them may provide more specific management strategies and may lead to new therapies in the future.
While more work is needed to further unravel the complex risk factors associated with asthma, ensuring that higher-risk patients are provided the appropriate care and the knowledge to help control their disease is a necessary step in improving the disparities in asthma care outcomes.
- Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report 2011; 32:1–14.
- National Institutes of Health. National Heart, Lung, and Blood Institute. Data Fact Sheet. Asthma statistics. January 1999. http://www.nhlbi.nih.gov/health/prof/lung/asthma/asthstat.pdf. Accessed February 1, 2012.
- Lang DM, Polansky M, Sherman MS. Hospitalizations for asthma in an urban population: 1995–1999. Ann Allergy Asthma Immunol 2009; 103:128–133.
- Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis 1990; 142:1351–1358.
- Koeppen-Schomerus G, Stevenson J, Plomin R. Genes and environment in asthma: a study of 4 year old twins. Arch Dis Child 2001; 85:398–400.
- Meng JF, Rosenwasser LJ. Unraveling the genetic basis of asthma and allergic diseases. Allergy Asthma Immunol Res 2010; 2:215–227.
- Stevenson LA, Gergen PJ, Hoover DR, Rosenstreich D, Mannino DM, Matte TD. Sociodemographic correlates of indoor allergen sensitivity among United States children. J Allergy Clin Immunol 2001; 108:747–752.
- Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med 1997; 336:1356–1363.
- Kobilka BK, Dixon RA, Frielle T, et al. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A 1987; 84:46–50.
- Maxwell TJ, Ameyaw MM, Pritchard S, et al. Beta-2 adrenergic receptor genotypes and haplotypes in different ethnic groups. Int J Mol Med 2005; 16:573–580.
- Israel E, Chinchilli VM, Ford JG, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet 2004; 364:1505–1512.
- Wells K, Pladevall M, Peterson EL, et al. Race-ethnic differences in factors associated with inhaled steroid adherence among adults with asthma. Am J Respir Crit Care Med 2008; 178:1194–1201.
- Carroll CL, Stoltz P, Schramm CM, Zucker AR. Beta2-adrenergic receptor polymorphisms affect response to treatment in children with severe asthma exacerbations. Chest 2009; 135:1186–1192.
- Bleecker ER, Nelson HS, Kraft M, et al. Beta2-receptor polymorphisms in patients receiving salmeterol with or without fluticasone propionate. Am J Respir Crit Care Med 2010; 181:676–687.
- Finkelstein Y, Bournissen FG, Hutson JR, Shannon M. Polymorphism of the ADRB2 gene and response to inhaled beta-agonists in children with asthma: a meta-analysis. J Asthma 2009; 46:900–905.
- Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A polymorphism in the 5’ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol 1999; 20:976–983.
- Kedda MA, Lose F, Duffy D, Bell E, Thompson PJ, Upham J. The CD14 C-159T polymorphism is not associated with asthma or asthma severity in an Australian adult population. Thorax 2005; 60:211–214.
- Zambelli-Weiner A, Ehrlich E, Stockton ML, et al. Evaluation of the CD14/-260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J Allergy Clin Immunol 2005; 115:1203–1209.
- Perzanowski MS, Miller RL, Thorne PS, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol 2006; 117:1082–1089.
- Williams LK, Oliver J, Peterson EL, et al. Gene-environment interactions between CD14 C-260T and endotoxin exposure on Foxp3+ and Foxp3− CD4+ lymphocyte numbers and total serum IgE levels in early childhood. Ann Allergy Asthma Immunol 2008; 100:128–136.
- Barnes KC. Ancestry, ancestry-informative markers, asthma, and the quest for personalized medicine. J Allergy Clin Immunol 2010; 126:1139–1140.
- Vergara C, Caraballo L, Mercado D, et al. African ancestry is associated with risk of asthma and high total serum IgE in a population from the Caribbean Coast of Colombia. Hum Genet 2009; 125:565–579.
- Gould W, Peterson EL, Karungi G, et al. Factors predicting inhaled corticosteroid responsiveness in African American patients with asthma. J Allergy Clin Immunol 2010; 126:1131–1138.
- Lang DM, Polansky M. Patterns of asthma mortality in Philadelphia from 1969 to 1991. N Engl J Med 1994; 331:1542–1546.
- Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. Am Rev Respir Dis 1990; 142:555–562.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. J Allergy Clin Immunol 2007; 120( suppl 5):S94–S138.
- Newacheck PW, Hughes DC, Stoddard JJ. Children’s access to primary care: differences by race, income, and insurance status. Pediatrics 1996; 97:26–32.
- Halfon N, Newacheck PW. Childhood asthma and poverty: differential impacts and utilization of health services. Pediatrics 1993; 91:56–61.
- Burt CW, Knapp DE. Ambulatory care visits for asthma: United States, 1993–94. Adv Data 1996; 277:1.
- Lieu TA, Lozano P, Finkelstein JA, et al. Racial/ethnic variation in asthma status and management practices among children in managed Medicaid. Pediatrics 2002; 109:857–865.
- Zoratti EM, Havstad S, Rodriguez J, Robens-Paradise Y, Lafata JE, McCarthy B. Health service use by African Americans and Caucasians with asthma in a managed care setting. Am J Respir Crit Care Med 1998; 158:371–377.
- Wisnivesky JP, Leventhal H, Halm EA. Predictors of asthma-related health care utilization and quality of life among inner-city patients with asthma. J Allergy Clin Immunol 2005; 116:636–642.
- Halm EA, Wisnivesky JP, Leventhal H. Quality and access to care among a cohort of inner-city adults with asthma: who gets guideline concordant care? Chest 2005; 128:1943–1950.
- Krishnan JA, Diette GB, Skinner EA, Clark BD, Steinwachs D, Wu AW. Race and sex differences in consistency of care with national asthma guidelines in managed care organizations. Arch Intern Med 2001; 161:1660–1668.
- Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 2002; 94:666–668.
- Boudreaux ED, Emond SD, Clark S, Camargo CA; Multicenter Airway Research Collaboration Investigators. Race/ethnicity and asthma among children presenting to the emergency department: differences in disease severity and management. Pediatrics 2003; 111:e615–e621.
- Trochtenberg DS, BeLue R, Piphus S, Washington N. Differing reports of asthma symptoms in African Americans and Caucasians. J Asthma 2008; 45:165–170.
- Hardie GE, Janson S, Gold WM, Carrieri-Kohlman V, Boushey HA. Ethnic differences: word descriptors used by African-American and white asthma patients during induced bronchoconstriction. Chest 2000; 117:935–943.
- Clark NM, Levison MJ, Evans D, Wasilewski Y, Feldman CH, Mellins RB. Communication within low income families and the management of asthma. Patient Educ Couns 1990; 15:191–210.
- Rhee H, Belyea MJ, Brasch J. Family support and asthma outcomes in adolescents: barriers to adherence as a mediator. J Adolesc Health 2010; 47:472–478.
- Quinn K, Kaufman JS, Siddiqi A, Yeatts KB. Parent perceptions of neighborhood stressors are associated with general health and child respiratory health among low-income, urban families. J Asthma 2010; 47:281–289.
- Loerbroks A, Apfelbacher CJ, Bosch JA, Stürmer T. Depressive symptoms, social support, and risk of adult asthma in a population-based cohort study. Psychosom Med 2010; 72:309–315.
- Wood DL, Valdez RB, Hayashi T, Shen A. Health of homeless children and housed, poor children. Pediatrics 1990; 86:858–866.
- O’Donnell L, Stueve A, Myint-U A. Parenting and violence toward self, partners, and others among inner-city young adults. Am J Public Health 2009; 99:2255–2260.
- Leventhal T, Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull 2000; 126:309–337.
- Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci U S A 2009; 106:12406–12411.
- Wright RJ, Mitchell H, Visness CM, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. Am J Public Health 2004; 94:625–632.
- George M, Freedman TG, Norfleet AL, Feldman HI, Apter AJ. Qualitative research-enhanced understanding of patients’ beliefs: results of focus groups with low-income, urban, African American adults with asthma. J Allergy Clin Immunol 2003; 111:967–973.
- Laster N, Holsey CN, Shendell DG, Mccarty FA, Celano M. Barriers to asthma management among urban families: caregiver and child perspectives. J Asthma 2009; 46:731–739.
- Lieu TA, Finkelstein JA, Lozano P, et al. Cultural competence policies and other predictors of asthma care quality for Medicaid-insured children. Pediatrics 2004; 114:e102–e110.
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004; 42:200–209.
- Bender B, Wamboldt FS, O’Connor SL, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol 2000; 85:416–421.
- Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol 2004; 114:1288–1293.
- Apter AJ, Boston RC, George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol 2003; 111:1219–1226.
- Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med 1998; 157:1810–1817.
- Williams LK, Joseph CL, Peterson EL, et al. Race-ethnicity, crime, and other factors associated with adherence to inhaled corticosteroids. J Allergy Clin Immunol 2007; 119:168–175.
- O’Malley AS, Sheppard VB, Schwartz M, Mandelblatt J. The role of trust in use of preventive services among low-income African-American women. Prev Med 2004; 38:777–785.
- Okelo SO, Wu AW, Merriman B, Krishnan JA, Diette GB. Are physician estimates of asthma severity less accurate in black than in white patients? J Gen Intern Med 2007; 22:976–981.
- Kinney PL, Aggarwal M, Northridge ME, Janssen NA, Shepard P. Airborne concentrations of PM(2.5) and diesel exhaust particles on Harlem sidewalks: a community-based pilot study. Environ Health Perspect 2000; 108:213–218.
- O’Neill MS, Jerrett M, Kawachi I, et al; Workshop on Air Pollution and Socioeconomic Conditions. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect 2003; 111:1861–1870.
- Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis 1993; 147:826–831.
- Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect 1999; 107:489–493.
- Yu O, Sheppard L, Lumley T, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptoms of asthma in Seattle-area children enrolled in the CAMP study. Environ Health Perspect 2000; 108:1209–1214.
- Slaughter JC, Lumley T, Sheppard L, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Ann Allergy Asthma Immunol 2003; 91:346–353.
- Koenig JQ, Jansen K, Mar TF, et al. Measurement of offline exhaled nitric oxide in a study of community exposure to air pollution. Environ Health Perspect 2003; 111:1625–1629.
- Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58:1227–1232.
- Delva J, Tellez M, Finlayson TL, et al. Cigarette smoking among low-income African Americans: a serious public health problem. Am J Prev Med 2005; 29:218–220.
- McLeish AC, Zvolensky MJ. Asthma and cigarette smoking: a review of the empirical literature. J Asthma 2010; 47:345–361.
- Chaudhuri R, Livingston E, McMahon AD, Thomson L, Borland W, Thomson NC. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med 2003; 168:1308–1311.
- Wilson SE, Kahn RS, Khoury J, Lanphear BP. Racial differences in exposure to environmental tobacco smoke among children. Environ Health Perspect 2005; 113:362–367.
- Huss K, Rand CS, Butz AM, et al. Home environmental risk factors in urban minority asthmatic children. Ann Allergy 1994; 72:173–177.
- Samir S, Colin Y, Thomas S. Impact of environmental tobacco smoke on children admitted with status asthmaticus in the pediatric intensive care unit. Pediatr Pulmonol 2010. [Epub ahead of print]
- Lannerö E, Wickman M, Pershagen G, Nordvall L. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE). Respir Res 2006; 7:3.
- Hedman L, Bjerg A, Sundberg S, Forsberg B, Rönmark E. Both environmental tobacco smoke and personal smoking is related to asthma and wheeze in teenagers. Thorax 2011; 66:20–25.
- Zhang G, Hayden CM, Khoo SK, et al. Beta2-adrenoceptor polymorphisms and asthma phenotypes: interactions with passive smoking. Eur Respir J 2007; 30:48–55.
- Rosenbaum E, Friedman S. The Housing Divide: How Generations of Immigrants Fare in New York’s Housing Market. New York, NY: New York University Press; 2007.
- Rosenbaum E. Racial/ethnic differences in asthma prevalence: the role of housing and neighborhood environments. J Health Soc Behav 2008; 49:131–145.
- Rauh VA, Chew GR, Garfinkel RS. Deteriorated housing contributes to high cockroach allergen levels in inner-city households. Environ Health Perspect 2002; 110( suppl 2):323–327.
- Eggleston PA, Rosenstreich D, Lynn H, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol 1998; 102:563–570.
- Togias A, Horowitz E, Joyner D, Guydon L, Malveaux F. Evaluating the factors that relate to asthma severity in adolescents. Int Arch Allergy Immunol 1997; 113:87–95.
- Alp H, Yu BH, Grant EN, Rao V, Moy JN. Cockroach allergy appears early in life in inner-city children with recurrent wheezing. Ann Allergy Asthma Immunol 2001; 86:51–54.
- Miller RL, Chew GL, Bell CA, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med 2001; 164:995–1001.
- Morgan WJ, Crain EF, Gruchalla RS, et al; Inner-City Asthma Study Group. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004; 351:1068–1080.
- Centers for Disease Control and Prevention (CDC). Overweight and Obesity. US Obesity Trends. http://templatelab.com/us-obesity-trends/. Accessed February 1, 2012.
- Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007; 175:661–666.
- Visness CM, London SJ, Daniels JL, et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. J Asthma 2010; 47:822–829.
- Camargo CA, Sutherland ER, Bailey W, et al. Effect of increased body mass index on asthma risk, impairment and response to asthma controller therapy in African Americans. Curr Med Res Opin 2010; 26:1629–1635.
- Hakala K, Stenius-Aarniala B, Sovijärvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 2000; 118:1315–1321.
- Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 2000; 320:827–832.
- Dixon AE, Holguin F, Sood A, et al; American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc 2010; 7:325–335.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999; 159:179–187.
- Harik-Khan RI, Muller DC, Wise RA. Racial difference in lung function in African-American and white children: effect of anthropometric, socioeconomic, nutritional, and environmental factors. Am J Epidemiol 2004; 160:893–900.
The last several decades have seen a dramatic surge in the prevalence of asthma. In 2009, there were an estimated 17.5 million adults and almost 7.1 million children with asthma in the United States,1 up from 9.5 million adults and slightly more than 5 million children in 1995.2
Multiple factors contribute to these disparities, including genetics, socioeconomic factors, cultural factors, health maintenance behaviors, provider-patient communication, air quality, and obesity.
GENETICS: 70% OF DESTINY?
The trend towards personalized medicine has spurred extensive research into the genetics of asthma. Studies in twins and familial aggregation studies suggest genetics plays a significant role, with estimates of the heritability of asthma as high as 70%.4,5 More than 100 candidate genes have been shown to be associated with asthma and atopy, 30 of them in five or more independent studies.6
Researchers face many challenges when investigating the genetics involved in asthma for a particular race. Race is both a biologic and a social construct and, as such, is a poor substitute for genetics. Race constitutes not only genetic differences in individuals, but also the behaviors, beliefs, and experiences that vary among races.
The clinical disease—the phenotype—is the product of the interaction of genes and these differing behaviors and exposures. Genetics can affect how environmental factors found in association with socioeconomic factors relate to asthma morbidity and mortality.
For example, as we will discuss below, African Americans are more likely than whites to be sensitized to cockroach allergen, even after controlling for socioeconomic variables that may be associated with greater exposure.7 High-level exposure to cockroach allergen in sensitized children has been associated with poor asthma outcomes.8 This suggests that a genetic difference may exist between African Americans and whites with respect to the potential to develop cockroach sensitization, and this difference may be of particular importance for those African Americans living in areas with higher levels of cockroach exposure.
Two polymorphisms
Two polymorphisms have garnered attention for their influence on African Americans with asthma:
TheADRB2gene. This gene codes for the beta-2 adrenergic receptor and resides at chromosome 5q13.9 The receptor is found on several types of cells in the lung, including airway smooth muscle and epithelial cells, and is responsible for the salutary effects of inhaled beta-2 agonists such as albuterol (eg, Proventil).
Allelic polymorphisms of this gene are clinically relevant. The substitution of arginine (Arg) for glycine (Gly) at codon 16 of this gene is responsible for differences in response to short-acting beta-2 agonists. The allelic frequency of Arg16 is lower in white Americans (39.3%) than in African Americans (49.2%), and thus African Americans are more likely to be homozygous for Arg16 (ie, to have the Arg/Arg genotype).10
People who are homozygous for Arg16 who use albuterol on a regular basis are at higher risk of untoward asthma outcomes.11 This is important, for several reasons. In general, adherence to inhaled corticosteroids is poor (not only in African Americans),12 and patients who do not take their inhaled corticosteroids as they should may rely on short-acting beta-2 agonists more frequently. Furthermore, African Americans may have a poorer response to the repeated doses of albuterol that are typically given in the emergency department and in the hospital for severe asthma exacerbations.13 Additionally, data suggest that Arg/Arg individuals have more frequent exacerbations independent of beta-agonist use,14 although curiously, patients who are homozygous for Arg16 have a greater benefit from single doses of short-acting beta-2 agonists than those who are Gly16 homozygous.15
TheCD14gene. An interesting relationship between innate immunity and asthma has recently been described. Polymorphisms of CD14, which codes for a receptor for endotoxin, have been uncovered. The single-nucleotide polymorphism variant thymine (T) at position −260 has been found in greater frequency in whites than in African Americans, who are more likely to have the cytosine (C) allele.16 An association between the CC genotype and atopy has been reported,16 although this has not been consistent.17
A possible explanation for these inconsistencies may lie in complex gene-environment interactions. The amount of endotoxin exposure may play a role in phenotypic expression. Individuals with the CC genotype were at lower risk of developing atopy when exposed to high levels of endotoxin; however, when exposed to lower levels of endotoxin, the CC genotype was associated with a higher risk of atopy.18 Nonfarm homes in westernized countries tend to have lower levels of endotoxin than farm homes, even in low-income urban areas.19 This implies that individuals with the CC allele, who are more likely to be African American, would be at greater risk for atopy in the United States. Greater knowledge of these types of gene-environment interactions may lead to improved understanding of the observations that have generated controversy concerning the “hygiene hypothesis.”
The details of how microbial exposure can influence the human immune response to antigen exposure are still being elucidated.20
These examples highlight not only the importance of genetics in the development of asthma, but also the role genes play in variation of treatment response and subsequent risk of morbidity and death. An understanding of these genetic differences among patients is clearly important for moving towards personalized treatment strategies for asthma.
Ancestry-informative markers
A developing strategy to assess the differences in asthma prevalence, severity, and response to treatment between racial groups is the use of ancestry-informative markers (AIMs).
AIMs are single-nucleotide polymorphisms that occur in varying allelic frequencies between ancestral groups, eg, continental Africans or European whites.21 AIMs provide an estimate of an individual’s proportion of ancestry—ie, of how “African” an African American is genetically.
African ancestry, determined using AIMs, was found to be associated with asthma in people living on the Caribbean coast of Colombia.22 However, one study found that AIMs could not predict an individual’s response to inhaled corticosteroids.23
Further research is necessary to find a technique to determine how groups of individuals can be characterized more precisely and managed more appropriately.
SOCIOECONOMIC FACTORS
African Americans living in low-income urban areas have an even greater prevalence of asthma and a greater risk of asthma-related morbidity and death than African Americans overall.3,24,25 Urban areas typically have a high proportion of residents living at or below the poverty level, and minorities often constitute a substantial proportion of the population in these areas. Evidence suggests that both African American race and lower socioeconomic status are independent risk factors for asthma prevalence, morbidity, and death.3,25
To provide better care for African Americans living in low-income urban areas, it is important to understand the factors that may be contributing to the higher morbidity and mortality rates in low-income urban areas.
Inadequate follow-up
Proper and routine follow-up for evaluation of asthma symptoms is essential for appropriate management. The Expert Panel Report 3 (EPR-3) of the National Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma,26 published in 2007, recommends that patients be seen at least every 6 months if they have been experiencing good control. While gaining control, patients should be seen every 2 to 6 weeks.26
Despite these recommendations, numerous studies have suggested that African Americans do not receive adequate follow-up. Children who are poor, are nonwhite or Hispanic, or are underinsured are more likely to lack routine health care27 and, more specifically, routine asthma care.28 Low-income patients are also more likely to receive care in a large hospital-run clinic or neighborhood clinic,27,28 where continuity of care may be less than ideal.29 Even among patients enrolled in Medicaid or Medicare, African American children with a primary care provider have fewer asthma visits compared with white Medicaid-insured children.30
Insufficient follow-up care contributes to greater asthma morbidity, resulting in, for example, more emergency department visits for asthma in African Americans.27,31,32
Suboptimal care
Data also suggest that the quality of care that residents of low-income urban areas receive is often suboptimal. Many people living in low-income urban areas are not provided with the knowledge and tools to treat asthma exacerbations at home.33 African Americans are also less likely to be seen by an asthma specialist31,34 as recommended for those with moderate or severe asthma.26
The EPR-3 guidelines also stress the importance of inhaled corticosteroids as the preferred therapy for all patients with persistent asthma. Even after controlling for the number of primary care visits, insurance status, and disease severity, African Americans are less likely to receive a prescription for inhaled corticosteroids, or they receive the same dosage of inhaled corticosteroids in the face of more severe disease.31,33,35,36
The reasons for these differences in treatment are not fully understood but are likely multiple. Lack of access to an asthma specialist and financial or formulary constraints are some of the potential barriers to optimal asthma care outcomes.
Misdiagnosis in the acute setting may also be a source of less-than-ideal care, as patients seen in emergency departments may be misdiagnosed with viral infection or bronchitis.
African Americans may report different symptoms than whites
Intriguing studies suggest that African Americans report different symptoms while describing asthma exacerbations.
In one study, compared with whites, African Americans were less likely to report nocturnal symptoms, dyspnea, or chest pain during exacerbations.37 In another study, when given a methacholine challenge that induced a significant drop in forced expiratory volume in 1 second (FEV1), African Americans with asthma were more likely to complain of upper airway symptoms as opposed to lower airway symptoms, compared with white patients.38
The symptoms that African Americans describe, such as having a tight throat or voice, are not typically regarded as related to asthma; for this reason, such descriptions may be an obstacle to correct diagnosis, management, and follow-up.
Asthma care providers should be aware of these observations to ensure that their patients are managed appropriately.
Lack of social support
Living in a low-income urban area presents many challenges that can interfere with proper asthma control.
Asthma diagnosis, management, and morbidity are affected by family support.39 Patients with asthma who lack sufficient financial support for treatment, who lack adequate psychological support, and who have more major life stressors are at higher risk of untoward outcomes. Disruption and dysfunction of the family and the supports available have been associated with greater asthma morbidity.40–42 Unfortunately, these types of stressors are all too common in families living in low-income urban areas.43–45
Multiple stressors that can occur more often in low-income urban areas, including exposure to violent crime, have also been linked to greater asthma morbidity.45–47
POOR PHYSICIAN-PATIENT COMMUNICATION
A consistent theme in focus groups of African Americans living in inner-city areas is the perception that health care providers are not effectively communicating and taking the time to listen to their concerns.48,49 Respondents believed they had better insight into their illness than their providers, and for this reason were better able to manage their disease.
The importance of an optimal provider-patient relationship was highlighted by a prospective cohort study in which Medicaid children receiving care at physician’s offices with the highest cultural competency scores were more adherent with their asthma controller medications.50
MEDICATION ADHERENCE RATES ARE DISTURBINGLY LOW
Rates of medication adherence for chronic diseases is disturbingly low, and may be even worse for pulmonary diseases.51 Reported rates of adherence to asthma medications among all patients range from 50% to 60%.52,53 Several studies showed that African Americans have a lower rate of adherence than do whites,53–55 even after adjusting for multiple socioeconomic variables.56
Many explanations have been proposed for this discrepancy, and all likely play a role in particular environments. For example, the incidence of crime in the surrounding area was inversely related to medication adherence after adjusting for socioeconomic factors.57 African Americans may have more concern about side effects associated with inhaled corticosteroid use and may be less likely to understand how these drugs work.52,53 A poor provider-patient relationship has also been cited as a barrier to adherence.55,57 Finally, physicians are more likely to underestimate asthma severity in an African American patient than in a white patient.58
Taking the time to ensure that patients truly understand all aspects of their disease and establishing a health care environment that is culturally appropriate may have a significant impact in patients with asthma.
ENVIRONMENTAL EXPOSURES
Air quality contributes to the greater asthma morbidity observed in urban residents, including African Americans. While poor outdoor air quality has not been clearly linked to a higher incidence of asthma, it has been associated with greater asthma morbidity. Poor air quality may affect individuals of all races, but with respect to ambient pollutants such as particulate matter and diesel exhaust, outdoor air quality is worse in urban environments where greater proportions of people of low socioeconomic status reside.59,60
The most extensively studied components of air pollution are ozone, sulfur dioxide, and particulate matter. These pollutants have been associated with a higher rate of emergency department visits,61,62 worse asthma symptoms,63,64 and higher exhaled nitric oxide levels.65
Tobacco smoke
Despite the substantial success of smoking cessation efforts nationwide, exposure to tobacco smoke continues to be common and is a significant risk factor for poor asthma control. Recent data suggest that African Americans and whites have a similar prevalence of smoking,66 but a study found a very high prevalence in low-income African Americans.67
Active smoking has been associated with worse asthma control and a higher risk of death.68 People with asthma who smoke are less likely to improve in their lung function and symptom scores when treated with short courses of oral glucocorticoids compared with both nonsmokers and former smokers.69
Secondhand smoke hurts too. Many children living in low-income urban areas are exposed to secondhand smoke or environmental tobacco smoke.70,71 Passive exposure in children has been associated with worse asthma outcomes, and data suggest such exposure may be a cause of asthma.68,72–74
Environmental tobacco smoke has also been implicated in gene-environment interactions. Patients who are either homozygous or heterozygous for the Arg allele at codon 16 of the ADRB2 gene (discussed above) had significantly lower FEV1 and forced vital capacity (FVC) values when exposed to passive tobacco smoke. This difference was not seen in people who were not exposed.75
Cockroach allergen
The type and condition of a person’s housing also plays a role in asthma-related morbidity and death. Across several socioeconomic levels, it has been suggested that African Americans have poorer-quality housing compared with whites.76 Some of the conditions found in low-quality houses, such as interruptions in heat, plumbing leaks, and the presence of rodents, have been associated with a higher prevalence of asthma in the household.77
Cockroach allergen exposure and sensitization is a major contributor to asthma morbidity in African Americans, particularly those living in poorer urban areas where cockroach allergen may be the most common indoor allergen.8 Living in older housing in urban areas is associated with higher exposure to cockroach allergen, and with subsequent sensitization.78,79 Exposure to high levels of the major cockroach allergen, Bla g 1, in sensitized individuals has been linked to a greater risk of hospitalization and unscheduled medical visits for asthma. This was not found to be the case for other common indoor allergens, such as dust mite and cat dander.8
However, it is not only exposure to high cockroach allergen levels that puts African Americans at risk. African Americans living in low-income urban areas may also be more likely than whites living in low-income urban areas to become sensitized to cockroach allergen.7,80 This suggests a gene-environment interaction that may be unique to African Americans. Moreover, cockroach sensitization may occur early in life.81,82
While successful cockroach avoidance measures and environmental control may be challenging, such measures have been shown to decrease rates of asthma morbidity.83
OBESITY
Obesity has been linked to an ever-growing list of diseases, one of which is asthma. Obesity is not a unique challenge for African Americans, but recent data from the US Centers for Disease Control and Prevention show that African Americans have a 51% higher prevalence of obesity compared with whites.84
Obesity is a risk factor for greater asthma morbidity and is a significant challenge in the African American community. The rise in obesity rates has paralleled the rise in asthma in recent decades. The higher one’s body mass index, the higher one’s risk of asthma.85 This association appears to be stronger in people without concurrent atopic disease.86 Obesity has also been associated with a poorer response to inhaled corticosteroids and a higher risk of asthma exacerbations.87 Interestingly, significant weight loss has been associated with improvements in both asthma control and lung function.88,89
What is the mechanism?
The underlying pathogenic mechanisms have not been completely elucidated, and they are likely multiple.
Adipokines (cytokines secreted by adipocytes) have been implicated. Two of the most extensively studied adipokines are leptin and adiponectin. Leptin production is increased in obesity, and it has inflammatory effects on both the innate and adaptive immune systems.90 The opposite is true for adiponectin, which may have anti-inflammatory properties and which decreases as the body mass index increases.90 The precise role these molecules may have in lung disease is undergoing further investigation.
Mechanical alterations in lung function may also contribute. Obese people have a lower functional residual capacity and expiratory reserve volume. Breathing with a lower-volume functional residual capacity results in decreased airway diameter and contributes to increased airway resistance.90 The decreased airway diameter may alter the contractile properties of airway smooth muscle and lead to increased airway responsiveness.90 These differences are in addition to the lower mean values of common spirometry indices such as the FEV1 and FVC, found in nonasthmatic African Americans compared with whites.91
Data suggest these differences are primarily due to anthropometric factors, with nutritional and environmental factors playing a less significant role.92 On this basis, the American Thoracic Society recommends applying race-specific reference standards for use with spirometry in order to accurately gauge lung function in African Americans.
APPROPRIATE CARE AND EDUCATION
The cause of greater asthma prevalence and severity among African Americans is multifactorial. It is likely that a number of factors work together, rather than separately, in influencing the development of asthma and its course.
Some risk factors are avoidable, and it is important to identify and ameliorate them. Others are not preventable, but knowledge of them may provide more specific management strategies and may lead to new therapies in the future.
While more work is needed to further unravel the complex risk factors associated with asthma, ensuring that higher-risk patients are provided the appropriate care and the knowledge to help control their disease is a necessary step in improving the disparities in asthma care outcomes.
The last several decades have seen a dramatic surge in the prevalence of asthma. In 2009, there were an estimated 17.5 million adults and almost 7.1 million children with asthma in the United States,1 up from 9.5 million adults and slightly more than 5 million children in 1995.2
Multiple factors contribute to these disparities, including genetics, socioeconomic factors, cultural factors, health maintenance behaviors, provider-patient communication, air quality, and obesity.
GENETICS: 70% OF DESTINY?
The trend towards personalized medicine has spurred extensive research into the genetics of asthma. Studies in twins and familial aggregation studies suggest genetics plays a significant role, with estimates of the heritability of asthma as high as 70%.4,5 More than 100 candidate genes have been shown to be associated with asthma and atopy, 30 of them in five or more independent studies.6
Researchers face many challenges when investigating the genetics involved in asthma for a particular race. Race is both a biologic and a social construct and, as such, is a poor substitute for genetics. Race constitutes not only genetic differences in individuals, but also the behaviors, beliefs, and experiences that vary among races.
The clinical disease—the phenotype—is the product of the interaction of genes and these differing behaviors and exposures. Genetics can affect how environmental factors found in association with socioeconomic factors relate to asthma morbidity and mortality.
For example, as we will discuss below, African Americans are more likely than whites to be sensitized to cockroach allergen, even after controlling for socioeconomic variables that may be associated with greater exposure.7 High-level exposure to cockroach allergen in sensitized children has been associated with poor asthma outcomes.8 This suggests that a genetic difference may exist between African Americans and whites with respect to the potential to develop cockroach sensitization, and this difference may be of particular importance for those African Americans living in areas with higher levels of cockroach exposure.
Two polymorphisms
Two polymorphisms have garnered attention for their influence on African Americans with asthma:
TheADRB2gene. This gene codes for the beta-2 adrenergic receptor and resides at chromosome 5q13.9 The receptor is found on several types of cells in the lung, including airway smooth muscle and epithelial cells, and is responsible for the salutary effects of inhaled beta-2 agonists such as albuterol (eg, Proventil).
Allelic polymorphisms of this gene are clinically relevant. The substitution of arginine (Arg) for glycine (Gly) at codon 16 of this gene is responsible for differences in response to short-acting beta-2 agonists. The allelic frequency of Arg16 is lower in white Americans (39.3%) than in African Americans (49.2%), and thus African Americans are more likely to be homozygous for Arg16 (ie, to have the Arg/Arg genotype).10
People who are homozygous for Arg16 who use albuterol on a regular basis are at higher risk of untoward asthma outcomes.11 This is important, for several reasons. In general, adherence to inhaled corticosteroids is poor (not only in African Americans),12 and patients who do not take their inhaled corticosteroids as they should may rely on short-acting beta-2 agonists more frequently. Furthermore, African Americans may have a poorer response to the repeated doses of albuterol that are typically given in the emergency department and in the hospital for severe asthma exacerbations.13 Additionally, data suggest that Arg/Arg individuals have more frequent exacerbations independent of beta-agonist use,14 although curiously, patients who are homozygous for Arg16 have a greater benefit from single doses of short-acting beta-2 agonists than those who are Gly16 homozygous.15
TheCD14gene. An interesting relationship between innate immunity and asthma has recently been described. Polymorphisms of CD14, which codes for a receptor for endotoxin, have been uncovered. The single-nucleotide polymorphism variant thymine (T) at position −260 has been found in greater frequency in whites than in African Americans, who are more likely to have the cytosine (C) allele.16 An association between the CC genotype and atopy has been reported,16 although this has not been consistent.17
A possible explanation for these inconsistencies may lie in complex gene-environment interactions. The amount of endotoxin exposure may play a role in phenotypic expression. Individuals with the CC genotype were at lower risk of developing atopy when exposed to high levels of endotoxin; however, when exposed to lower levels of endotoxin, the CC genotype was associated with a higher risk of atopy.18 Nonfarm homes in westernized countries tend to have lower levels of endotoxin than farm homes, even in low-income urban areas.19 This implies that individuals with the CC allele, who are more likely to be African American, would be at greater risk for atopy in the United States. Greater knowledge of these types of gene-environment interactions may lead to improved understanding of the observations that have generated controversy concerning the “hygiene hypothesis.”
The details of how microbial exposure can influence the human immune response to antigen exposure are still being elucidated.20
These examples highlight not only the importance of genetics in the development of asthma, but also the role genes play in variation of treatment response and subsequent risk of morbidity and death. An understanding of these genetic differences among patients is clearly important for moving towards personalized treatment strategies for asthma.
Ancestry-informative markers
A developing strategy to assess the differences in asthma prevalence, severity, and response to treatment between racial groups is the use of ancestry-informative markers (AIMs).
AIMs are single-nucleotide polymorphisms that occur in varying allelic frequencies between ancestral groups, eg, continental Africans or European whites.21 AIMs provide an estimate of an individual’s proportion of ancestry—ie, of how “African” an African American is genetically.
African ancestry, determined using AIMs, was found to be associated with asthma in people living on the Caribbean coast of Colombia.22 However, one study found that AIMs could not predict an individual’s response to inhaled corticosteroids.23
Further research is necessary to find a technique to determine how groups of individuals can be characterized more precisely and managed more appropriately.
SOCIOECONOMIC FACTORS
African Americans living in low-income urban areas have an even greater prevalence of asthma and a greater risk of asthma-related morbidity and death than African Americans overall.3,24,25 Urban areas typically have a high proportion of residents living at or below the poverty level, and minorities often constitute a substantial proportion of the population in these areas. Evidence suggests that both African American race and lower socioeconomic status are independent risk factors for asthma prevalence, morbidity, and death.3,25
To provide better care for African Americans living in low-income urban areas, it is important to understand the factors that may be contributing to the higher morbidity and mortality rates in low-income urban areas.
Inadequate follow-up
Proper and routine follow-up for evaluation of asthma symptoms is essential for appropriate management. The Expert Panel Report 3 (EPR-3) of the National Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma,26 published in 2007, recommends that patients be seen at least every 6 months if they have been experiencing good control. While gaining control, patients should be seen every 2 to 6 weeks.26
Despite these recommendations, numerous studies have suggested that African Americans do not receive adequate follow-up. Children who are poor, are nonwhite or Hispanic, or are underinsured are more likely to lack routine health care27 and, more specifically, routine asthma care.28 Low-income patients are also more likely to receive care in a large hospital-run clinic or neighborhood clinic,27,28 where continuity of care may be less than ideal.29 Even among patients enrolled in Medicaid or Medicare, African American children with a primary care provider have fewer asthma visits compared with white Medicaid-insured children.30
Insufficient follow-up care contributes to greater asthma morbidity, resulting in, for example, more emergency department visits for asthma in African Americans.27,31,32
Suboptimal care
Data also suggest that the quality of care that residents of low-income urban areas receive is often suboptimal. Many people living in low-income urban areas are not provided with the knowledge and tools to treat asthma exacerbations at home.33 African Americans are also less likely to be seen by an asthma specialist31,34 as recommended for those with moderate or severe asthma.26
The EPR-3 guidelines also stress the importance of inhaled corticosteroids as the preferred therapy for all patients with persistent asthma. Even after controlling for the number of primary care visits, insurance status, and disease severity, African Americans are less likely to receive a prescription for inhaled corticosteroids, or they receive the same dosage of inhaled corticosteroids in the face of more severe disease.31,33,35,36
The reasons for these differences in treatment are not fully understood but are likely multiple. Lack of access to an asthma specialist and financial or formulary constraints are some of the potential barriers to optimal asthma care outcomes.
Misdiagnosis in the acute setting may also be a source of less-than-ideal care, as patients seen in emergency departments may be misdiagnosed with viral infection or bronchitis.
African Americans may report different symptoms than whites
Intriguing studies suggest that African Americans report different symptoms while describing asthma exacerbations.
In one study, compared with whites, African Americans were less likely to report nocturnal symptoms, dyspnea, or chest pain during exacerbations.37 In another study, when given a methacholine challenge that induced a significant drop in forced expiratory volume in 1 second (FEV1), African Americans with asthma were more likely to complain of upper airway symptoms as opposed to lower airway symptoms, compared with white patients.38
The symptoms that African Americans describe, such as having a tight throat or voice, are not typically regarded as related to asthma; for this reason, such descriptions may be an obstacle to correct diagnosis, management, and follow-up.
Asthma care providers should be aware of these observations to ensure that their patients are managed appropriately.
Lack of social support
Living in a low-income urban area presents many challenges that can interfere with proper asthma control.
Asthma diagnosis, management, and morbidity are affected by family support.39 Patients with asthma who lack sufficient financial support for treatment, who lack adequate psychological support, and who have more major life stressors are at higher risk of untoward outcomes. Disruption and dysfunction of the family and the supports available have been associated with greater asthma morbidity.40–42 Unfortunately, these types of stressors are all too common in families living in low-income urban areas.43–45
Multiple stressors that can occur more often in low-income urban areas, including exposure to violent crime, have also been linked to greater asthma morbidity.45–47
POOR PHYSICIAN-PATIENT COMMUNICATION
A consistent theme in focus groups of African Americans living in inner-city areas is the perception that health care providers are not effectively communicating and taking the time to listen to their concerns.48,49 Respondents believed they had better insight into their illness than their providers, and for this reason were better able to manage their disease.
The importance of an optimal provider-patient relationship was highlighted by a prospective cohort study in which Medicaid children receiving care at physician’s offices with the highest cultural competency scores were more adherent with their asthma controller medications.50
MEDICATION ADHERENCE RATES ARE DISTURBINGLY LOW
Rates of medication adherence for chronic diseases is disturbingly low, and may be even worse for pulmonary diseases.51 Reported rates of adherence to asthma medications among all patients range from 50% to 60%.52,53 Several studies showed that African Americans have a lower rate of adherence than do whites,53–55 even after adjusting for multiple socioeconomic variables.56
Many explanations have been proposed for this discrepancy, and all likely play a role in particular environments. For example, the incidence of crime in the surrounding area was inversely related to medication adherence after adjusting for socioeconomic factors.57 African Americans may have more concern about side effects associated with inhaled corticosteroid use and may be less likely to understand how these drugs work.52,53 A poor provider-patient relationship has also been cited as a barrier to adherence.55,57 Finally, physicians are more likely to underestimate asthma severity in an African American patient than in a white patient.58
Taking the time to ensure that patients truly understand all aspects of their disease and establishing a health care environment that is culturally appropriate may have a significant impact in patients with asthma.
ENVIRONMENTAL EXPOSURES
Air quality contributes to the greater asthma morbidity observed in urban residents, including African Americans. While poor outdoor air quality has not been clearly linked to a higher incidence of asthma, it has been associated with greater asthma morbidity. Poor air quality may affect individuals of all races, but with respect to ambient pollutants such as particulate matter and diesel exhaust, outdoor air quality is worse in urban environments where greater proportions of people of low socioeconomic status reside.59,60
The most extensively studied components of air pollution are ozone, sulfur dioxide, and particulate matter. These pollutants have been associated with a higher rate of emergency department visits,61,62 worse asthma symptoms,63,64 and higher exhaled nitric oxide levels.65
Tobacco smoke
Despite the substantial success of smoking cessation efforts nationwide, exposure to tobacco smoke continues to be common and is a significant risk factor for poor asthma control. Recent data suggest that African Americans and whites have a similar prevalence of smoking,66 but a study found a very high prevalence in low-income African Americans.67
Active smoking has been associated with worse asthma control and a higher risk of death.68 People with asthma who smoke are less likely to improve in their lung function and symptom scores when treated with short courses of oral glucocorticoids compared with both nonsmokers and former smokers.69
Secondhand smoke hurts too. Many children living in low-income urban areas are exposed to secondhand smoke or environmental tobacco smoke.70,71 Passive exposure in children has been associated with worse asthma outcomes, and data suggest such exposure may be a cause of asthma.68,72–74
Environmental tobacco smoke has also been implicated in gene-environment interactions. Patients who are either homozygous or heterozygous for the Arg allele at codon 16 of the ADRB2 gene (discussed above) had significantly lower FEV1 and forced vital capacity (FVC) values when exposed to passive tobacco smoke. This difference was not seen in people who were not exposed.75
Cockroach allergen
The type and condition of a person’s housing also plays a role in asthma-related morbidity and death. Across several socioeconomic levels, it has been suggested that African Americans have poorer-quality housing compared with whites.76 Some of the conditions found in low-quality houses, such as interruptions in heat, plumbing leaks, and the presence of rodents, have been associated with a higher prevalence of asthma in the household.77
Cockroach allergen exposure and sensitization is a major contributor to asthma morbidity in African Americans, particularly those living in poorer urban areas where cockroach allergen may be the most common indoor allergen.8 Living in older housing in urban areas is associated with higher exposure to cockroach allergen, and with subsequent sensitization.78,79 Exposure to high levels of the major cockroach allergen, Bla g 1, in sensitized individuals has been linked to a greater risk of hospitalization and unscheduled medical visits for asthma. This was not found to be the case for other common indoor allergens, such as dust mite and cat dander.8
However, it is not only exposure to high cockroach allergen levels that puts African Americans at risk. African Americans living in low-income urban areas may also be more likely than whites living in low-income urban areas to become sensitized to cockroach allergen.7,80 This suggests a gene-environment interaction that may be unique to African Americans. Moreover, cockroach sensitization may occur early in life.81,82
While successful cockroach avoidance measures and environmental control may be challenging, such measures have been shown to decrease rates of asthma morbidity.83
OBESITY
Obesity has been linked to an ever-growing list of diseases, one of which is asthma. Obesity is not a unique challenge for African Americans, but recent data from the US Centers for Disease Control and Prevention show that African Americans have a 51% higher prevalence of obesity compared with whites.84
Obesity is a risk factor for greater asthma morbidity and is a significant challenge in the African American community. The rise in obesity rates has paralleled the rise in asthma in recent decades. The higher one’s body mass index, the higher one’s risk of asthma.85 This association appears to be stronger in people without concurrent atopic disease.86 Obesity has also been associated with a poorer response to inhaled corticosteroids and a higher risk of asthma exacerbations.87 Interestingly, significant weight loss has been associated with improvements in both asthma control and lung function.88,89
What is the mechanism?
The underlying pathogenic mechanisms have not been completely elucidated, and they are likely multiple.
Adipokines (cytokines secreted by adipocytes) have been implicated. Two of the most extensively studied adipokines are leptin and adiponectin. Leptin production is increased in obesity, and it has inflammatory effects on both the innate and adaptive immune systems.90 The opposite is true for adiponectin, which may have anti-inflammatory properties and which decreases as the body mass index increases.90 The precise role these molecules may have in lung disease is undergoing further investigation.
Mechanical alterations in lung function may also contribute. Obese people have a lower functional residual capacity and expiratory reserve volume. Breathing with a lower-volume functional residual capacity results in decreased airway diameter and contributes to increased airway resistance.90 The decreased airway diameter may alter the contractile properties of airway smooth muscle and lead to increased airway responsiveness.90 These differences are in addition to the lower mean values of common spirometry indices such as the FEV1 and FVC, found in nonasthmatic African Americans compared with whites.91
Data suggest these differences are primarily due to anthropometric factors, with nutritional and environmental factors playing a less significant role.92 On this basis, the American Thoracic Society recommends applying race-specific reference standards for use with spirometry in order to accurately gauge lung function in African Americans.
APPROPRIATE CARE AND EDUCATION
The cause of greater asthma prevalence and severity among African Americans is multifactorial. It is likely that a number of factors work together, rather than separately, in influencing the development of asthma and its course.
Some risk factors are avoidable, and it is important to identify and ameliorate them. Others are not preventable, but knowledge of them may provide more specific management strategies and may lead to new therapies in the future.
While more work is needed to further unravel the complex risk factors associated with asthma, ensuring that higher-risk patients are provided the appropriate care and the knowledge to help control their disease is a necessary step in improving the disparities in asthma care outcomes.
- Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report 2011; 32:1–14.
- National Institutes of Health. National Heart, Lung, and Blood Institute. Data Fact Sheet. Asthma statistics. January 1999. http://www.nhlbi.nih.gov/health/prof/lung/asthma/asthstat.pdf. Accessed February 1, 2012.
- Lang DM, Polansky M, Sherman MS. Hospitalizations for asthma in an urban population: 1995–1999. Ann Allergy Asthma Immunol 2009; 103:128–133.
- Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis 1990; 142:1351–1358.
- Koeppen-Schomerus G, Stevenson J, Plomin R. Genes and environment in asthma: a study of 4 year old twins. Arch Dis Child 2001; 85:398–400.
- Meng JF, Rosenwasser LJ. Unraveling the genetic basis of asthma and allergic diseases. Allergy Asthma Immunol Res 2010; 2:215–227.
- Stevenson LA, Gergen PJ, Hoover DR, Rosenstreich D, Mannino DM, Matte TD. Sociodemographic correlates of indoor allergen sensitivity among United States children. J Allergy Clin Immunol 2001; 108:747–752.
- Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med 1997; 336:1356–1363.
- Kobilka BK, Dixon RA, Frielle T, et al. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A 1987; 84:46–50.
- Maxwell TJ, Ameyaw MM, Pritchard S, et al. Beta-2 adrenergic receptor genotypes and haplotypes in different ethnic groups. Int J Mol Med 2005; 16:573–580.
- Israel E, Chinchilli VM, Ford JG, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet 2004; 364:1505–1512.
- Wells K, Pladevall M, Peterson EL, et al. Race-ethnic differences in factors associated with inhaled steroid adherence among adults with asthma. Am J Respir Crit Care Med 2008; 178:1194–1201.
- Carroll CL, Stoltz P, Schramm CM, Zucker AR. Beta2-adrenergic receptor polymorphisms affect response to treatment in children with severe asthma exacerbations. Chest 2009; 135:1186–1192.
- Bleecker ER, Nelson HS, Kraft M, et al. Beta2-receptor polymorphisms in patients receiving salmeterol with or without fluticasone propionate. Am J Respir Crit Care Med 2010; 181:676–687.
- Finkelstein Y, Bournissen FG, Hutson JR, Shannon M. Polymorphism of the ADRB2 gene and response to inhaled beta-agonists in children with asthma: a meta-analysis. J Asthma 2009; 46:900–905.
- Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A polymorphism in the 5’ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol 1999; 20:976–983.
- Kedda MA, Lose F, Duffy D, Bell E, Thompson PJ, Upham J. The CD14 C-159T polymorphism is not associated with asthma or asthma severity in an Australian adult population. Thorax 2005; 60:211–214.
- Zambelli-Weiner A, Ehrlich E, Stockton ML, et al. Evaluation of the CD14/-260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J Allergy Clin Immunol 2005; 115:1203–1209.
- Perzanowski MS, Miller RL, Thorne PS, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol 2006; 117:1082–1089.
- Williams LK, Oliver J, Peterson EL, et al. Gene-environment interactions between CD14 C-260T and endotoxin exposure on Foxp3+ and Foxp3− CD4+ lymphocyte numbers and total serum IgE levels in early childhood. Ann Allergy Asthma Immunol 2008; 100:128–136.
- Barnes KC. Ancestry, ancestry-informative markers, asthma, and the quest for personalized medicine. J Allergy Clin Immunol 2010; 126:1139–1140.
- Vergara C, Caraballo L, Mercado D, et al. African ancestry is associated with risk of asthma and high total serum IgE in a population from the Caribbean Coast of Colombia. Hum Genet 2009; 125:565–579.
- Gould W, Peterson EL, Karungi G, et al. Factors predicting inhaled corticosteroid responsiveness in African American patients with asthma. J Allergy Clin Immunol 2010; 126:1131–1138.
- Lang DM, Polansky M. Patterns of asthma mortality in Philadelphia from 1969 to 1991. N Engl J Med 1994; 331:1542–1546.
- Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. Am Rev Respir Dis 1990; 142:555–562.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. J Allergy Clin Immunol 2007; 120( suppl 5):S94–S138.
- Newacheck PW, Hughes DC, Stoddard JJ. Children’s access to primary care: differences by race, income, and insurance status. Pediatrics 1996; 97:26–32.
- Halfon N, Newacheck PW. Childhood asthma and poverty: differential impacts and utilization of health services. Pediatrics 1993; 91:56–61.
- Burt CW, Knapp DE. Ambulatory care visits for asthma: United States, 1993–94. Adv Data 1996; 277:1.
- Lieu TA, Lozano P, Finkelstein JA, et al. Racial/ethnic variation in asthma status and management practices among children in managed Medicaid. Pediatrics 2002; 109:857–865.
- Zoratti EM, Havstad S, Rodriguez J, Robens-Paradise Y, Lafata JE, McCarthy B. Health service use by African Americans and Caucasians with asthma in a managed care setting. Am J Respir Crit Care Med 1998; 158:371–377.
- Wisnivesky JP, Leventhal H, Halm EA. Predictors of asthma-related health care utilization and quality of life among inner-city patients with asthma. J Allergy Clin Immunol 2005; 116:636–642.
- Halm EA, Wisnivesky JP, Leventhal H. Quality and access to care among a cohort of inner-city adults with asthma: who gets guideline concordant care? Chest 2005; 128:1943–1950.
- Krishnan JA, Diette GB, Skinner EA, Clark BD, Steinwachs D, Wu AW. Race and sex differences in consistency of care with national asthma guidelines in managed care organizations. Arch Intern Med 2001; 161:1660–1668.
- Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 2002; 94:666–668.
- Boudreaux ED, Emond SD, Clark S, Camargo CA; Multicenter Airway Research Collaboration Investigators. Race/ethnicity and asthma among children presenting to the emergency department: differences in disease severity and management. Pediatrics 2003; 111:e615–e621.
- Trochtenberg DS, BeLue R, Piphus S, Washington N. Differing reports of asthma symptoms in African Americans and Caucasians. J Asthma 2008; 45:165–170.
- Hardie GE, Janson S, Gold WM, Carrieri-Kohlman V, Boushey HA. Ethnic differences: word descriptors used by African-American and white asthma patients during induced bronchoconstriction. Chest 2000; 117:935–943.
- Clark NM, Levison MJ, Evans D, Wasilewski Y, Feldman CH, Mellins RB. Communication within low income families and the management of asthma. Patient Educ Couns 1990; 15:191–210.
- Rhee H, Belyea MJ, Brasch J. Family support and asthma outcomes in adolescents: barriers to adherence as a mediator. J Adolesc Health 2010; 47:472–478.
- Quinn K, Kaufman JS, Siddiqi A, Yeatts KB. Parent perceptions of neighborhood stressors are associated with general health and child respiratory health among low-income, urban families. J Asthma 2010; 47:281–289.
- Loerbroks A, Apfelbacher CJ, Bosch JA, Stürmer T. Depressive symptoms, social support, and risk of adult asthma in a population-based cohort study. Psychosom Med 2010; 72:309–315.
- Wood DL, Valdez RB, Hayashi T, Shen A. Health of homeless children and housed, poor children. Pediatrics 1990; 86:858–866.
- O’Donnell L, Stueve A, Myint-U A. Parenting and violence toward self, partners, and others among inner-city young adults. Am J Public Health 2009; 99:2255–2260.
- Leventhal T, Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull 2000; 126:309–337.
- Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci U S A 2009; 106:12406–12411.
- Wright RJ, Mitchell H, Visness CM, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. Am J Public Health 2004; 94:625–632.
- George M, Freedman TG, Norfleet AL, Feldman HI, Apter AJ. Qualitative research-enhanced understanding of patients’ beliefs: results of focus groups with low-income, urban, African American adults with asthma. J Allergy Clin Immunol 2003; 111:967–973.
- Laster N, Holsey CN, Shendell DG, Mccarty FA, Celano M. Barriers to asthma management among urban families: caregiver and child perspectives. J Asthma 2009; 46:731–739.
- Lieu TA, Finkelstein JA, Lozano P, et al. Cultural competence policies and other predictors of asthma care quality for Medicaid-insured children. Pediatrics 2004; 114:e102–e110.
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004; 42:200–209.
- Bender B, Wamboldt FS, O’Connor SL, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol 2000; 85:416–421.
- Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol 2004; 114:1288–1293.
- Apter AJ, Boston RC, George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol 2003; 111:1219–1226.
- Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med 1998; 157:1810–1817.
- Williams LK, Joseph CL, Peterson EL, et al. Race-ethnicity, crime, and other factors associated with adherence to inhaled corticosteroids. J Allergy Clin Immunol 2007; 119:168–175.
- O’Malley AS, Sheppard VB, Schwartz M, Mandelblatt J. The role of trust in use of preventive services among low-income African-American women. Prev Med 2004; 38:777–785.
- Okelo SO, Wu AW, Merriman B, Krishnan JA, Diette GB. Are physician estimates of asthma severity less accurate in black than in white patients? J Gen Intern Med 2007; 22:976–981.
- Kinney PL, Aggarwal M, Northridge ME, Janssen NA, Shepard P. Airborne concentrations of PM(2.5) and diesel exhaust particles on Harlem sidewalks: a community-based pilot study. Environ Health Perspect 2000; 108:213–218.
- O’Neill MS, Jerrett M, Kawachi I, et al; Workshop on Air Pollution and Socioeconomic Conditions. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect 2003; 111:1861–1870.
- Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis 1993; 147:826–831.
- Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect 1999; 107:489–493.
- Yu O, Sheppard L, Lumley T, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptoms of asthma in Seattle-area children enrolled in the CAMP study. Environ Health Perspect 2000; 108:1209–1214.
- Slaughter JC, Lumley T, Sheppard L, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Ann Allergy Asthma Immunol 2003; 91:346–353.
- Koenig JQ, Jansen K, Mar TF, et al. Measurement of offline exhaled nitric oxide in a study of community exposure to air pollution. Environ Health Perspect 2003; 111:1625–1629.
- Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58:1227–1232.
- Delva J, Tellez M, Finlayson TL, et al. Cigarette smoking among low-income African Americans: a serious public health problem. Am J Prev Med 2005; 29:218–220.
- McLeish AC, Zvolensky MJ. Asthma and cigarette smoking: a review of the empirical literature. J Asthma 2010; 47:345–361.
- Chaudhuri R, Livingston E, McMahon AD, Thomson L, Borland W, Thomson NC. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med 2003; 168:1308–1311.
- Wilson SE, Kahn RS, Khoury J, Lanphear BP. Racial differences in exposure to environmental tobacco smoke among children. Environ Health Perspect 2005; 113:362–367.
- Huss K, Rand CS, Butz AM, et al. Home environmental risk factors in urban minority asthmatic children. Ann Allergy 1994; 72:173–177.
- Samir S, Colin Y, Thomas S. Impact of environmental tobacco smoke on children admitted with status asthmaticus in the pediatric intensive care unit. Pediatr Pulmonol 2010. [Epub ahead of print]
- Lannerö E, Wickman M, Pershagen G, Nordvall L. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE). Respir Res 2006; 7:3.
- Hedman L, Bjerg A, Sundberg S, Forsberg B, Rönmark E. Both environmental tobacco smoke and personal smoking is related to asthma and wheeze in teenagers. Thorax 2011; 66:20–25.
- Zhang G, Hayden CM, Khoo SK, et al. Beta2-adrenoceptor polymorphisms and asthma phenotypes: interactions with passive smoking. Eur Respir J 2007; 30:48–55.
- Rosenbaum E, Friedman S. The Housing Divide: How Generations of Immigrants Fare in New York’s Housing Market. New York, NY: New York University Press; 2007.
- Rosenbaum E. Racial/ethnic differences in asthma prevalence: the role of housing and neighborhood environments. J Health Soc Behav 2008; 49:131–145.
- Rauh VA, Chew GR, Garfinkel RS. Deteriorated housing contributes to high cockroach allergen levels in inner-city households. Environ Health Perspect 2002; 110( suppl 2):323–327.
- Eggleston PA, Rosenstreich D, Lynn H, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol 1998; 102:563–570.
- Togias A, Horowitz E, Joyner D, Guydon L, Malveaux F. Evaluating the factors that relate to asthma severity in adolescents. Int Arch Allergy Immunol 1997; 113:87–95.
- Alp H, Yu BH, Grant EN, Rao V, Moy JN. Cockroach allergy appears early in life in inner-city children with recurrent wheezing. Ann Allergy Asthma Immunol 2001; 86:51–54.
- Miller RL, Chew GL, Bell CA, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med 2001; 164:995–1001.
- Morgan WJ, Crain EF, Gruchalla RS, et al; Inner-City Asthma Study Group. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004; 351:1068–1080.
- Centers for Disease Control and Prevention (CDC). Overweight and Obesity. US Obesity Trends. http://templatelab.com/us-obesity-trends/. Accessed February 1, 2012.
- Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007; 175:661–666.
- Visness CM, London SJ, Daniels JL, et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. J Asthma 2010; 47:822–829.
- Camargo CA, Sutherland ER, Bailey W, et al. Effect of increased body mass index on asthma risk, impairment and response to asthma controller therapy in African Americans. Curr Med Res Opin 2010; 26:1629–1635.
- Hakala K, Stenius-Aarniala B, Sovijärvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 2000; 118:1315–1321.
- Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 2000; 320:827–832.
- Dixon AE, Holguin F, Sood A, et al; American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc 2010; 7:325–335.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999; 159:179–187.
- Harik-Khan RI, Muller DC, Wise RA. Racial difference in lung function in African-American and white children: effect of anthropometric, socioeconomic, nutritional, and environmental factors. Am J Epidemiol 2004; 160:893–900.
- Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report 2011; 32:1–14.
- National Institutes of Health. National Heart, Lung, and Blood Institute. Data Fact Sheet. Asthma statistics. January 1999. http://www.nhlbi.nih.gov/health/prof/lung/asthma/asthstat.pdf. Accessed February 1, 2012.
- Lang DM, Polansky M, Sherman MS. Hospitalizations for asthma in an urban population: 1995–1999. Ann Allergy Asthma Immunol 2009; 103:128–133.
- Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis 1990; 142:1351–1358.
- Koeppen-Schomerus G, Stevenson J, Plomin R. Genes and environment in asthma: a study of 4 year old twins. Arch Dis Child 2001; 85:398–400.
- Meng JF, Rosenwasser LJ. Unraveling the genetic basis of asthma and allergic diseases. Allergy Asthma Immunol Res 2010; 2:215–227.
- Stevenson LA, Gergen PJ, Hoover DR, Rosenstreich D, Mannino DM, Matte TD. Sociodemographic correlates of indoor allergen sensitivity among United States children. J Allergy Clin Immunol 2001; 108:747–752.
- Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med 1997; 336:1356–1363.
- Kobilka BK, Dixon RA, Frielle T, et al. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A 1987; 84:46–50.
- Maxwell TJ, Ameyaw MM, Pritchard S, et al. Beta-2 adrenergic receptor genotypes and haplotypes in different ethnic groups. Int J Mol Med 2005; 16:573–580.
- Israel E, Chinchilli VM, Ford JG, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet 2004; 364:1505–1512.
- Wells K, Pladevall M, Peterson EL, et al. Race-ethnic differences in factors associated with inhaled steroid adherence among adults with asthma. Am J Respir Crit Care Med 2008; 178:1194–1201.
- Carroll CL, Stoltz P, Schramm CM, Zucker AR. Beta2-adrenergic receptor polymorphisms affect response to treatment in children with severe asthma exacerbations. Chest 2009; 135:1186–1192.
- Bleecker ER, Nelson HS, Kraft M, et al. Beta2-receptor polymorphisms in patients receiving salmeterol with or without fluticasone propionate. Am J Respir Crit Care Med 2010; 181:676–687.
- Finkelstein Y, Bournissen FG, Hutson JR, Shannon M. Polymorphism of the ADRB2 gene and response to inhaled beta-agonists in children with asthma: a meta-analysis. J Asthma 2009; 46:900–905.
- Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A polymorphism in the 5’ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol 1999; 20:976–983.
- Kedda MA, Lose F, Duffy D, Bell E, Thompson PJ, Upham J. The CD14 C-159T polymorphism is not associated with asthma or asthma severity in an Australian adult population. Thorax 2005; 60:211–214.
- Zambelli-Weiner A, Ehrlich E, Stockton ML, et al. Evaluation of the CD14/-260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J Allergy Clin Immunol 2005; 115:1203–1209.
- Perzanowski MS, Miller RL, Thorne PS, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol 2006; 117:1082–1089.
- Williams LK, Oliver J, Peterson EL, et al. Gene-environment interactions between CD14 C-260T and endotoxin exposure on Foxp3+ and Foxp3− CD4+ lymphocyte numbers and total serum IgE levels in early childhood. Ann Allergy Asthma Immunol 2008; 100:128–136.
- Barnes KC. Ancestry, ancestry-informative markers, asthma, and the quest for personalized medicine. J Allergy Clin Immunol 2010; 126:1139–1140.
- Vergara C, Caraballo L, Mercado D, et al. African ancestry is associated with risk of asthma and high total serum IgE in a population from the Caribbean Coast of Colombia. Hum Genet 2009; 125:565–579.
- Gould W, Peterson EL, Karungi G, et al. Factors predicting inhaled corticosteroid responsiveness in African American patients with asthma. J Allergy Clin Immunol 2010; 126:1131–1138.
- Lang DM, Polansky M. Patterns of asthma mortality in Philadelphia from 1969 to 1991. N Engl J Med 1994; 331:1542–1546.
- Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. Am Rev Respir Dis 1990; 142:555–562.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. J Allergy Clin Immunol 2007; 120( suppl 5):S94–S138.
- Newacheck PW, Hughes DC, Stoddard JJ. Children’s access to primary care: differences by race, income, and insurance status. Pediatrics 1996; 97:26–32.
- Halfon N, Newacheck PW. Childhood asthma and poverty: differential impacts and utilization of health services. Pediatrics 1993; 91:56–61.
- Burt CW, Knapp DE. Ambulatory care visits for asthma: United States, 1993–94. Adv Data 1996; 277:1.
- Lieu TA, Lozano P, Finkelstein JA, et al. Racial/ethnic variation in asthma status and management practices among children in managed Medicaid. Pediatrics 2002; 109:857–865.
- Zoratti EM, Havstad S, Rodriguez J, Robens-Paradise Y, Lafata JE, McCarthy B. Health service use by African Americans and Caucasians with asthma in a managed care setting. Am J Respir Crit Care Med 1998; 158:371–377.
- Wisnivesky JP, Leventhal H, Halm EA. Predictors of asthma-related health care utilization and quality of life among inner-city patients with asthma. J Allergy Clin Immunol 2005; 116:636–642.
- Halm EA, Wisnivesky JP, Leventhal H. Quality and access to care among a cohort of inner-city adults with asthma: who gets guideline concordant care? Chest 2005; 128:1943–1950.
- Krishnan JA, Diette GB, Skinner EA, Clark BD, Steinwachs D, Wu AW. Race and sex differences in consistency of care with national asthma guidelines in managed care organizations. Arch Intern Med 2001; 161:1660–1668.
- Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 2002; 94:666–668.
- Boudreaux ED, Emond SD, Clark S, Camargo CA; Multicenter Airway Research Collaboration Investigators. Race/ethnicity and asthma among children presenting to the emergency department: differences in disease severity and management. Pediatrics 2003; 111:e615–e621.
- Trochtenberg DS, BeLue R, Piphus S, Washington N. Differing reports of asthma symptoms in African Americans and Caucasians. J Asthma 2008; 45:165–170.
- Hardie GE, Janson S, Gold WM, Carrieri-Kohlman V, Boushey HA. Ethnic differences: word descriptors used by African-American and white asthma patients during induced bronchoconstriction. Chest 2000; 117:935–943.
- Clark NM, Levison MJ, Evans D, Wasilewski Y, Feldman CH, Mellins RB. Communication within low income families and the management of asthma. Patient Educ Couns 1990; 15:191–210.
- Rhee H, Belyea MJ, Brasch J. Family support and asthma outcomes in adolescents: barriers to adherence as a mediator. J Adolesc Health 2010; 47:472–478.
- Quinn K, Kaufman JS, Siddiqi A, Yeatts KB. Parent perceptions of neighborhood stressors are associated with general health and child respiratory health among low-income, urban families. J Asthma 2010; 47:281–289.
- Loerbroks A, Apfelbacher CJ, Bosch JA, Stürmer T. Depressive symptoms, social support, and risk of adult asthma in a population-based cohort study. Psychosom Med 2010; 72:309–315.
- Wood DL, Valdez RB, Hayashi T, Shen A. Health of homeless children and housed, poor children. Pediatrics 1990; 86:858–866.
- O’Donnell L, Stueve A, Myint-U A. Parenting and violence toward self, partners, and others among inner-city young adults. Am J Public Health 2009; 99:2255–2260.
- Leventhal T, Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull 2000; 126:309–337.
- Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci U S A 2009; 106:12406–12411.
- Wright RJ, Mitchell H, Visness CM, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. Am J Public Health 2004; 94:625–632.
- George M, Freedman TG, Norfleet AL, Feldman HI, Apter AJ. Qualitative research-enhanced understanding of patients’ beliefs: results of focus groups with low-income, urban, African American adults with asthma. J Allergy Clin Immunol 2003; 111:967–973.
- Laster N, Holsey CN, Shendell DG, Mccarty FA, Celano M. Barriers to asthma management among urban families: caregiver and child perspectives. J Asthma 2009; 46:731–739.
- Lieu TA, Finkelstein JA, Lozano P, et al. Cultural competence policies and other predictors of asthma care quality for Medicaid-insured children. Pediatrics 2004; 114:e102–e110.
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004; 42:200–209.
- Bender B, Wamboldt FS, O’Connor SL, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol 2000; 85:416–421.
- Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol 2004; 114:1288–1293.
- Apter AJ, Boston RC, George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol 2003; 111:1219–1226.
- Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med 1998; 157:1810–1817.
- Williams LK, Joseph CL, Peterson EL, et al. Race-ethnicity, crime, and other factors associated with adherence to inhaled corticosteroids. J Allergy Clin Immunol 2007; 119:168–175.
- O’Malley AS, Sheppard VB, Schwartz M, Mandelblatt J. The role of trust in use of preventive services among low-income African-American women. Prev Med 2004; 38:777–785.
- Okelo SO, Wu AW, Merriman B, Krishnan JA, Diette GB. Are physician estimates of asthma severity less accurate in black than in white patients? J Gen Intern Med 2007; 22:976–981.
- Kinney PL, Aggarwal M, Northridge ME, Janssen NA, Shepard P. Airborne concentrations of PM(2.5) and diesel exhaust particles on Harlem sidewalks: a community-based pilot study. Environ Health Perspect 2000; 108:213–218.
- O’Neill MS, Jerrett M, Kawachi I, et al; Workshop on Air Pollution and Socioeconomic Conditions. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect 2003; 111:1861–1870.
- Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis 1993; 147:826–831.
- Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect 1999; 107:489–493.
- Yu O, Sheppard L, Lumley T, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptoms of asthma in Seattle-area children enrolled in the CAMP study. Environ Health Perspect 2000; 108:1209–1214.
- Slaughter JC, Lumley T, Sheppard L, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Ann Allergy Asthma Immunol 2003; 91:346–353.
- Koenig JQ, Jansen K, Mar TF, et al. Measurement of offline exhaled nitric oxide in a study of community exposure to air pollution. Environ Health Perspect 2003; 111:1625–1629.
- Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58:1227–1232.
- Delva J, Tellez M, Finlayson TL, et al. Cigarette smoking among low-income African Americans: a serious public health problem. Am J Prev Med 2005; 29:218–220.
- McLeish AC, Zvolensky MJ. Asthma and cigarette smoking: a review of the empirical literature. J Asthma 2010; 47:345–361.
- Chaudhuri R, Livingston E, McMahon AD, Thomson L, Borland W, Thomson NC. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med 2003; 168:1308–1311.
- Wilson SE, Kahn RS, Khoury J, Lanphear BP. Racial differences in exposure to environmental tobacco smoke among children. Environ Health Perspect 2005; 113:362–367.
- Huss K, Rand CS, Butz AM, et al. Home environmental risk factors in urban minority asthmatic children. Ann Allergy 1994; 72:173–177.
- Samir S, Colin Y, Thomas S. Impact of environmental tobacco smoke on children admitted with status asthmaticus in the pediatric intensive care unit. Pediatr Pulmonol 2010. [Epub ahead of print]
- Lannerö E, Wickman M, Pershagen G, Nordvall L. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE). Respir Res 2006; 7:3.
- Hedman L, Bjerg A, Sundberg S, Forsberg B, Rönmark E. Both environmental tobacco smoke and personal smoking is related to asthma and wheeze in teenagers. Thorax 2011; 66:20–25.
- Zhang G, Hayden CM, Khoo SK, et al. Beta2-adrenoceptor polymorphisms and asthma phenotypes: interactions with passive smoking. Eur Respir J 2007; 30:48–55.
- Rosenbaum E, Friedman S. The Housing Divide: How Generations of Immigrants Fare in New York’s Housing Market. New York, NY: New York University Press; 2007.
- Rosenbaum E. Racial/ethnic differences in asthma prevalence: the role of housing and neighborhood environments. J Health Soc Behav 2008; 49:131–145.
- Rauh VA, Chew GR, Garfinkel RS. Deteriorated housing contributes to high cockroach allergen levels in inner-city households. Environ Health Perspect 2002; 110( suppl 2):323–327.
- Eggleston PA, Rosenstreich D, Lynn H, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol 1998; 102:563–570.
- Togias A, Horowitz E, Joyner D, Guydon L, Malveaux F. Evaluating the factors that relate to asthma severity in adolescents. Int Arch Allergy Immunol 1997; 113:87–95.
- Alp H, Yu BH, Grant EN, Rao V, Moy JN. Cockroach allergy appears early in life in inner-city children with recurrent wheezing. Ann Allergy Asthma Immunol 2001; 86:51–54.
- Miller RL, Chew GL, Bell CA, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med 2001; 164:995–1001.
- Morgan WJ, Crain EF, Gruchalla RS, et al; Inner-City Asthma Study Group. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004; 351:1068–1080.
- Centers for Disease Control and Prevention (CDC). Overweight and Obesity. US Obesity Trends. http://templatelab.com/us-obesity-trends/. Accessed February 1, 2012.
- Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007; 175:661–666.
- Visness CM, London SJ, Daniels JL, et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. J Asthma 2010; 47:822–829.
- Camargo CA, Sutherland ER, Bailey W, et al. Effect of increased body mass index on asthma risk, impairment and response to asthma controller therapy in African Americans. Curr Med Res Opin 2010; 26:1629–1635.
- Hakala K, Stenius-Aarniala B, Sovijärvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 2000; 118:1315–1321.
- Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 2000; 320:827–832.
- Dixon AE, Holguin F, Sood A, et al; American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc 2010; 7:325–335.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999; 159:179–187.
- Harik-Khan RI, Muller DC, Wise RA. Racial difference in lung function in African-American and white children: effect of anthropometric, socioeconomic, nutritional, and environmental factors. Am J Epidemiol 2004; 160:893–900.
KEY POINTS
- To better identify those at risk, researchers are looking at genetic markers such as polymorphisms in ADRB2 and CD14.
- Exposure to tobacco smoke and to cockroach allergen contribute to higher rates of asthma prevalence and morbidity.
- African Americans are more likely to receive suboptimal care, in particular to be misdiagnosed with other conditions, to not receive inhaled corticosteroids, and to not receive proper follow-up.
- Better physician-patient communication is one of the keys to improving this problem.