User login
Persistent organic pollutants (POPs), endocrine-disrupting, lipophilic chemicals that concentrate in adipose tissue, increasingly are being studied for a wide range of health effects.1 Persistent organic pollutants include bisphenol A, phthalates, dioxins, hexachlorobenzene, dichlorodiphenyltrichloroethane (DDT), polybrominated diphenyl ethers, and polychlorinated biphenyls (PCBs). Chlorinated dibenzo-p-dioxins are known as polychlorinated dibenzodioxins (PCDDs), or simply dioxins. Categorization of this group of chemicals is based on the structural chlorinated constituents. Of the 75 congener molecules, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic, and this dioxin, given its more serious health implications, has been studied the most.1,2
Because it was a contaminant in the herbicide Agent Orange, the main defoliant used by the US military in southern Vietnam during the Vietnam War, TCDD is of primary interest. Agent Orange consists of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in equal parts. Like other dioxins, TCDD is lipophilic and retained in adipose tissue.1,3 Contemporaneous sources include occupational and residential exposure from pulp and paper mills, metallurgy, incinerators, industrial waste, fossil fuel combustion, and industrial accidents and poisonings.1-4
Another main class of POPs, polychlorinated benzenes, includes 209 synthetic PCB congener chemicals, a subset of which is referred to as dioxin-like PCBs.1 Organochlorine (OC) pesticides and PCBs were once manufactured as lubricants and coolants for electronics but are now banned; nevertheless, they remain concentrated in fish and mammals and persist in the food chain.3,5,6 These chemicals of interest (COIs) are graded for toxicity based on toxic equivalency factors relative to TCDDs in a 2005 World Health Organization assessment.3
Polychlorinated dibenzofurans (PCDFs), TCDD, PCBs, PCDDs, and other environmental toxins are being studied as possible contributing factors in the development of hypertension. The authors review the results of several recent studies on COI exposure and hypertension.
In 2017, the American College of Cardiology and the American Heart Association lowered the threshold for hypertension to systolic blood pressure (SBP) > 130 mm Hg and diastolic blood pressure (DBP) > 80 mm Hg.7 This new guideline would categorize 46% of the US population as having hypertension, compared with 32% under the former cutoff of 140/90 mm Hg.7 Modifiable factors (eg, diet, body mass index [BMI], smoking, alcohol, physical activity) and nonmodifiable factors (eg, age, family history, sex, race/ethnicity) have a role in the pathophysiology of hypertension. Between 90% and 95% of hypertension is considered primary. Hypertension increases the risk of developing ischemic heart disease, atherosclerosis, aortic dissection, small blood vessel disease, congestive heart failure, and renal failure, and thus results in considerable morbidity and mortality each year.8
Contaminant Exposure and Hypertension Risk
Vietnam-Era Army Chemical Corps
The US Army Chemical Corps (ACC) Vietnam-Era Veterans Health Study (2012-2013) recorded the long-term health burdens imposed by Agent Orange exposure and Vietnam War service.9,10 This cross-sectional study reexamined a subset of 5,609 Vietnam-era ACC veterans for an association of self-reported, physician-diagnosed hypertension (≥ 140/90 mm Hg) and herbicide spraying history confirmed with serum TCDD levels. The 22 Army units that made up the ACC were in charge of spreading Agent Orange and other defoliants on opposition camps between 1965 and 1973. The herbicide was dispersed aerially and on the ground. The ACC was also responsible for dispensing napalm, tear gas, and other chemicals.
A previous phone survey found an association of self-reported hypertension and herbicide spraying in ACC veterans with associated Vietnam service and herbicide spraying history, verified with serum TCDD levels (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.00-1.58).9 Median age of ACC veterans with Vietnam War service at the time of the survey was 53 years.
The 2012-2013 study assessed respondents with a record of their serum TCDD measurements from the time of the survey.10 Most of the respondents were aged in their 60s. The stated purpose of the health study was to examine the association of Vietnam veterans’ occupational herbicide exposure and hypertension risk, rather than isolate a certain responsible chemical, though serum TCDD levels were used to confirm spraying history. After adjustments for age, tobacco use, alcohol use, race, and BMI but not salt intake, family history of hypertension, psychiatric health, or diabetes mellitus (DM) comorbidity, the OR of self-reported, physician-diagnosed hypertension was 1.74 (95% CI, 1.44-2.11) for sprayers and 1.26 (95% CI, 1.05-1.53) for Vietnam veterans.10
Vietnam War Veterans From Korea
Soldiers of the Republic of Korea (ROK) who fought in the Vietnam War also were exposed to Agent Orange and other defoliants and herbicides. In 2013, Yi and colleagues contacted 187,897 ROK Vietnam veterans to analyze their Agent Orange exposure and self-reported diseases decades after the war.11 By mail, the researchers administered a questionnaire of perceived Agent Orange exposure (eg, spraying, handling spray equipment, having contact with COIs). The Korean veterans were classified by military assignment and by their proximity to areas sprayed with Agent Orange, according to the military records of 3 US combat units: Capital Division, 9th Division, and Marine Second Brigade. The ROK veterans in those units presumably would have similar levels of Agent Orange exposure.
The questionnaire response rate was 69%. The 114,562 respondents were divided into groups based on self-perceived exposure (no, low, moderate, high) and qualitative exposure level, derived from service history (battalion/company, division/brigade). After adjusting for BMI, smoking, alcohol use, physical activity, use of nonoccupational herbicides, education, income, and military rank, Yi and colleagues found a statistically significant association of hypertension and self-reported perceived Agent Orange exposure (P < .001) and a statistically significant association of hypertension and exposure in the division/brigade group with the highest exposure level (P < .001).11 The highest ORs were found for high- vs low-exposure and moderate- vs low-exposure subsets in self-reported perceived Agent Orange levels: 1.60 (95% CI, 1.56-1.65) and 1.70 (95% CI, 1.64-1.77), respectively. However, adjusted ORs in proximity-based exposure for all groups were > 1.03.
Inuits in Canada and Greenland
To study total PCBs, non-dioxin-like PCBs, OC pesticides, and their metabolites in plasma, public health researchers Valera and colleagues focused on the Inuit town of Nunavik (in Canada), where there is contamination from foods like fish, a mainstay of the Inuit diet.5 A health survey was sent to 400 households randomly selected from 1,378 households in 14 villages. Data were collected between September and December 1992. In total, 518 people between ages 18 and 74 years agreed to undergo a physical examination, and 492 agreed to have blood drawn. Laboratories measured serum PCB congeners and 13 chlorinated pesticides or their metabolites. Blood pressure (BP) was measured 3 times, and the last 2 measurements averaged. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg.
Of the 518 participants, 315 (134 men, 181 women) had complete BP, serum POP levels, and confounding variables recorded, and these were subsequently analyzed. Mean age was 32.7 years. Polychlorinated biphenyls congeners 105 and 118 were higher in women than in men; no other congeners were quantitatively different. Associations between POP levels and hypertension were analyzed with multiple logistic regression modeling, with adjustments for age, sex, fasting blood glucose, waist circumference, smoking, alcohol use, and physical activity, as well as the common contaminants lead, mercury, and omega-3 polyunsaturated fatty acids (n-3 PUFA).The researchers adjusted for n-3 PUFA because of the posited BP-lowering effects. Inuits consume large amounts of the polyunsaturated fatty acids DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid).5
Using congeners found in at least 70% of the total samples, the study authors found a statistically significant association between certain PCBs, both dioxin-like (DL-PCBs) and non-dioxin-like (NDL-PCBs), and increased risk of hypertension. Congeners 101, 105 (DL-PCB), 138, and 187, as well as p,p'-DDE, were also significantly associated with a higher risk of hypertension. Congener 99 was associated with increased SBP, and congener 118 (DL-PCB) was associated with increased SBP and DBP. Some congeners, such as the OC pesticides, p,p'-DDT, β-hexachlorocyclohexane, and oxychlordane, were inversely associated with hypertension.
In 2012, Valera and colleagues conducted a similar study of Greenland Inuits who also consume marine mammals and fish and present with high POP levels.6 Despite correcting for n-3 PUFA, they found no significant association involving DL-PCBs, NDL-PCBs, or OC pesticides.
Japanese Background Exposures
Nakamoto and colleagues conducted a cross-sectional study of 2,266 Japanese women and men who had been exposed to background (vs occupational or wartime) levels of dioxins, including PCDDs, PCDFs, and DL-PCBs.12 The dioxins likely originated from combustion of chlorinated materials and older manufactured electronics components. The study participants had lived in urban areas or in farming or fishing villages for at least 10 consecutive years and had no concomitant occupational exposure to dioxins. Mean (SD) age was 43.5 (13.6) years for the men and 45.3 (14.0) years for the women. Participants volunteered their disease histories, which included physician-diagnosed hypertension (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg). Logistic regression analyses were adjusted for BMI, sex, age, regional residential area, smoking, alcohol use, and survey year. In fishing regions, PCDDs, PCDFs, and DL-PCBs were significantly higher than in the other regions. Of the 2,266 participants, 647 reported physician-diagnosed hypertension. Dividing the toxic equivalents of serum COI levels into quartiles of concentration, Nakamoto and colleagues found a statistically significant association of hypertension and increased toxic equivalent levels of PCDDs, PCDFs, DL-PCBs, and total dioxins.
Italian Male Steelworkers
In a 2016 retrospective cohort study, Cappelletti and colleagues assessed the health burden of workers at a steel recycling plant in Trento, Italy. The plant, which had been using an electric arc furnace without a coke oven, had been exposing workers to dust containing PCBs, PCDDs, PCDFs, and other metals.13 Each hour, roughly 2 to 5 kg of dust was being released inside the plant (diffuse emissions), and exposure extended to a 2-km radius around the plant. A cohort of 331 plant workers, identified and assessed through company records, had been exposed to diffuse emissions for at least 1 year between 1979 and 2009. This group was compared with a control group of 32 office workers from that company, as identified by company records. The authors found a risk ratio (RR) of 2.23 in cases of noncomplicated hypertension and an RR of 2.01 in cases of complicated hypertension, defined as hypertension with organ damage.
Elderly in Sweden
In a study of 1,016 Swedish men and women who were aged 70 years or older and were living in Uppsala, Sweden, Lind and colleagues calculated average supine BP from 3 sphygmomanometer measurements after 30 minutes of rest.14 The researchers used high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) to measure the serum levels of a set of 23 POPs—16 PCB congeners, 5 OC pesticides, 1 brominated biphenyl ether congener, and octachloro-p-dibenzodioxin—and lipid-normalized the values. They used logistic regression to assess POP levels and prevalent hypertension (BP ≥ 140/90 mm Hg or use of antihypertensives), adjusting for sex, BMI, smoking status, exercise, and education. Among the COIs with the highest circulating lipid-normalized POP levels were PCB congeners 180, 138, and 170 and DDE. There was no clear relationship between toxic equivalents and hypertension; after multivariate adjustments, only DDE showed a statistically significant OR: 1.25 (95% CI, 1.07-1.47).
Organic Pollutants and Hypertension
Using National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2002, Ha and colleagues conducted a cross-sectional study of a 524-adult subset of patients who were exposed to background levels of POPs and had newly diagnosed hypertension (≥ 140/90 mm Hg).15 In the NHANES study, the CDC collected standardized patient history information, physical examination findings, and venous blood sample results. Recorded BP data points were the averages of 3 separate SBP and DBP readings from a sphygmomanometer, as recommended by the AHA. The NHANES study recorded POPs with HRGC/HRMS.
Ha and colleagues selected 12 POPs, and standardized the COI concentrations against lipid concentration. The lipid-standardized POP concentrations used were at a higher level of detection and found in at least 60% of the study patients. The researchers used a logistic regression model to calculate multivariate-adjusted OR separately in men and women, adjusting for race/ethnicity, smoking/alcohol use, physical activity, BMI, cotinine level, and income level. Among the 56 men and 67 women with newly diagnosed prevalent hypertension, PCDD levels in women were positively associated with hypertension but not correlated with higher or lower toxic equivalency factors. Dioxin and NDL-PCBs were positively associated with hypertension in men but negatively in women. Ha and colleagues postulated that this approach of studying a US population subset of patients with background exposure to POPs, instead of groups with high concentrations of exposure (eg, Vietnam War veterans or those exposed occupationally or in industrial accidents), provides an alternative observable effect of long-term, low-dose exposure of a blend of POPs.15
Discussion
In vivo and in vitro studies have found that dioxins induce a subset of 35 genes, including microsomal P450 enzymes, kinases and phosphates, and DNA repair proteins. A microarray profile of cardiovascular murine tissue and cultured vascular smooth muscle cells exposed to TCDD found known dioxin-inducible genes Cyp1b1, a phase 1 drug metabolism enzyme, and Aldh3A1, another drug metabolism gene up-regulated, among lectin-related natural killer cell receptor, insulin-like growth factor binding protein, and cyclin G2.16
Dioxins bind avidly to the aryl hydrocarbon receptor (AhR), a cytosolic transcription factor that also interacts with other xenobiotic compounds with varying affinities. TCDD is one of the most potent ligands for AhR, and other DL compounds have a lower binding affinity. AhR dimerizes in the nucleus with the AhR nuclear translocator and then binds genomic dioxin response elements and induces the expression of cytochrome P450 genes, such as CYP1A1.17
The AhRs are highly expressed in the vascular endothelium.17 Agbor and colleagues found that mice with endothelial AhR knockouts showed decreased baseline SBP and DBP.18 When challenged with angiotensin II, a potent vasoconstrictor, AhR-/- mice failed to show an increase in DBP. AhR-/- exhibited reduced ex vivo aortic contraction in the presence of angiotensin II in aortas with perivascular adipose tissue. Notably, compared with wild-type mice, AhR-/- mice had reduced renin-angiotensinsystem gene expression in the visceral adipose, linking the AhR receptor with the endogenous renin-angiotensin-aldosterone system (RAAS).
Early studies have shown that mice lacking AhR do not demonstrate TCDD toxicity.20 More recently, Kopf and colleagues found that TCDD exposure in mice led to increased BP and cardiac hypertrophy, possibly linked to increased superoxide production in the vasculature.21 When exposed to TCDD, mice showed enhanced CYP1A1 mRNA expression in the left ventricle, kidney, and aorta by day 35 and increased CYP1B1 mRNA expression in the left ventricle after 60 days. Within the first week of TCDD exposure, the mean arterial pressure for the exposure group was statistically significantly increased, showing a trend of peaks and plateaus. Mice exposed to TCDD also showed left ventricular concentric hypertrophy, which is typical of systemic hypertension.8,21 Kerley-Hamilton and colleagues found that AhR ligand activation increased atherosclerosis.22
Most hypertension is idiopathic. Research into the downstream effects of AhR suggests it induces vascular oxidative stress and increases atherosclerosis.22 It is unclear whether this is an initiating or synergistic factor in the development of hypertension. The study results described here indicate that dioxins initiate BP changes through the endothelial AhR receptor, but this mechanism has been proved only in an animal model. Ongoing studies are needed to examine the molecular changes in humans. Clinicians can be advised that dioxin exposure, rather than being an initiating factor, would at most contribute to an accumulating series of assaults, including genetics, lifestyle, and environmental factors, and that these assaults progress to hypertension only after passing a threshold.23 Moreover, many of the studies described here categorized hypertension under the guideline of 140/90 mm Hg. Future studies may use the newer guideline, which will affect their results.
Conclusion
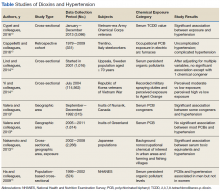
Studies have shown an association between dioxins and endocrine disruption, reproductive and developmental problems, and certain cancers.3,24 The Seveso Women’s Health Study of an industrial accident in Italy linked dioxins to an incidence of DM, obesity, or metabolic syndrome.25 By contrast, evidence of a link between dioxins and hypertension has been limited and inconsistent. Seven of the 8 studies reviewed in this study found moderate evidence of association in patients with at least 1 chemical congener and a certain subset of the study population (Table).
The Vietnam-Era Veterans Health Study found a higher OR of developing hypertension in herbicide sprayers than in its control group. Korean Vietnam War veterans stratified by either self-reported risk or military assignment also had significant associations. For male steelworkers in Italy, occupational exposure had a moderately higher RR in the exposure cohort. In the NHANES study, background levels of POPs were positively associated, but only in men. A nonoccupational study in urban and rural areas of Japan found a significant association between dioxins and hypertension. A nonoccupational study of elderly Swedes found a significant association with only 1 chemical congener. A study of Inuits in Greenland found no significant associations, but a study of Inuits in Canada did yield an association.Recent studies maintain the 2012 veterans update regarding a limited but suggestive association of dioxin and hypertension.4 Despite having high power because of the number of exposed patients, these observational studies can posit only an associative relationship, not a causal one. These studies also are limited by their categorization of dioxin exposure levels—ranging from perceived exposure to proximity and direct serum dioxin measurement. Moreover, chemical levels are measured an inconsistent number of years after exposure, and therefore, as dioxins are primarily metabolized by CYP genes, different metabolic rates could account for different susceptibility to health effects.2
In vivo animal studies could better characterize the effect of time point of exposure and effects on hypertension. Studies could also examine the synergistic effects of dioxins and other toxins, or smoking or alcohol use, on hypertension. New clinical guidelines for hypertension will have an impact on studies. Overall, clinicians who treat patients with known exposure to dioxins can suggest with moderate confidence that it is likely not a primary reason for the development of hypertension. At most, dioxin exposure is a contributing factor in the development of hypertension, with lifestyle, smoking, diet, and genetics playing more compelling roles.
1. Van den Berg M, Birnbaum L, Bosveld AT, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106(12):775-792.
2. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Koplan JP. Toxicological profile for chlorinated dibenzo-p-dioxins. https://www.atsdr.cdc .gov/toxprofiles/tp104.pdf. Published December 1998. Accessed April 3, 2018.
3. Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223-241.
4. Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Ninth Biennial Update); Board of the Health of Select Populations, Institute of Medicine. Veterans and Agent Orange: Update 2012. Washington, DC: National Academies Press; 2014.
5. Valera B, Ayotte P, Poirier P, Dewailly E. Associations between plasma persistent organic pollutant levels and blood pressure in Inuit adults from Nunavik. Environ Int. 2013;59:282-289.
6. Valera B, Jørgensen ME, Jeppesen C, Bjerregaard P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. 2013;122:65-73.
7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;Nov 13:pii:HYP.0000000000000066. [Epub ahead of print.]
8. Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier; 2014.
9. Kang HK, Dalager NA, Needham LL, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006;49(11):875-884.
10. Cypel YS, Kress AM, Eber SM, Schneiderman AI, Davey VJ. Herbicide exposure, Vietnam service, and hypertension risk in Army Chemical Corps veterans. J Occup Environ Med. 2016;58(11):1127-1136.
11. Yi SW, Ohrr H, Hong JS, Yi JJ. Agent Orange exposure and prevalence of self-reported diseases in Korean Vietnam veterans. J Prev Med Public Health. 2013;46(5):213-225.
12. Nakamoto M, Arisawa K, Uemura H, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health. 2013;86(8):849-859.
13. Cappelletti R, Ceppi M, Claudatus J, Gennaro V. Health status of male steel workers at an electric arc furnace (EAF) in Trentino, Italy. J Occup Med Toxicol. 2016;11:7.
14. Lind PM, Penell J, Salihovic S, van Bavel B, Lind L. Circulating levels of p,p’-DDE are related to prevalent hypertension in the elderly. Environ Res. 2014;129:27-31.
15. Ha MH, Lee DH, Son HK, Park SK, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999–2002. J Hum Hypertens. 2009;23(4):274-286.
16. Puga A, Sartor MA, Huang M, et al. Gene expression profiles of mouse aorta and cultured vascular smooth muscle cells differ widely, yet show common responses to dioxin exposure. Cardiovasc Toxicol. 2004;4(4):385-404.
17. Swanson HI, Bradfield CA. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993;3(5):213-230.
18. Agbor LN, Elased KM, Walker MK. Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness. Biochem Pharmacol. 2011;82(5):514-523.
19. Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338(1):311-317.
20. Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173-179.
21. Kopf PG, Scott JA, Agbor LN, et al. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117(2):537-546.
22. Kerley-Hamilton JS, Trask HW, Ridley CJ, et al. Inherent and benzo[a]pyrene-induced differential aryl hydrocarbon receptor signaling greatly affects life span, atherosclerosis, cardiac gene expression, and body and heart growth in mice. Toxicol Sci. 2012;126(2):391-404.
23. Narkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Pressure. 2005;14(2):69-71.
24. Kogevinas M. Human health effects of dioxins: cancer, reproductive and endocrine system effects. Hum Reprod Update. 2001;7(3):331-339.
25. Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso Women’s Health Study. Environ Health Perspect. 2013;121(8):906-911.
Persistent organic pollutants (POPs), endocrine-disrupting, lipophilic chemicals that concentrate in adipose tissue, increasingly are being studied for a wide range of health effects.1 Persistent organic pollutants include bisphenol A, phthalates, dioxins, hexachlorobenzene, dichlorodiphenyltrichloroethane (DDT), polybrominated diphenyl ethers, and polychlorinated biphenyls (PCBs). Chlorinated dibenzo-p-dioxins are known as polychlorinated dibenzodioxins (PCDDs), or simply dioxins. Categorization of this group of chemicals is based on the structural chlorinated constituents. Of the 75 congener molecules, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic, and this dioxin, given its more serious health implications, has been studied the most.1,2
Because it was a contaminant in the herbicide Agent Orange, the main defoliant used by the US military in southern Vietnam during the Vietnam War, TCDD is of primary interest. Agent Orange consists of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in equal parts. Like other dioxins, TCDD is lipophilic and retained in adipose tissue.1,3 Contemporaneous sources include occupational and residential exposure from pulp and paper mills, metallurgy, incinerators, industrial waste, fossil fuel combustion, and industrial accidents and poisonings.1-4
Another main class of POPs, polychlorinated benzenes, includes 209 synthetic PCB congener chemicals, a subset of which is referred to as dioxin-like PCBs.1 Organochlorine (OC) pesticides and PCBs were once manufactured as lubricants and coolants for electronics but are now banned; nevertheless, they remain concentrated in fish and mammals and persist in the food chain.3,5,6 These chemicals of interest (COIs) are graded for toxicity based on toxic equivalency factors relative to TCDDs in a 2005 World Health Organization assessment.3
Polychlorinated dibenzofurans (PCDFs), TCDD, PCBs, PCDDs, and other environmental toxins are being studied as possible contributing factors in the development of hypertension. The authors review the results of several recent studies on COI exposure and hypertension.
In 2017, the American College of Cardiology and the American Heart Association lowered the threshold for hypertension to systolic blood pressure (SBP) > 130 mm Hg and diastolic blood pressure (DBP) > 80 mm Hg.7 This new guideline would categorize 46% of the US population as having hypertension, compared with 32% under the former cutoff of 140/90 mm Hg.7 Modifiable factors (eg, diet, body mass index [BMI], smoking, alcohol, physical activity) and nonmodifiable factors (eg, age, family history, sex, race/ethnicity) have a role in the pathophysiology of hypertension. Between 90% and 95% of hypertension is considered primary. Hypertension increases the risk of developing ischemic heart disease, atherosclerosis, aortic dissection, small blood vessel disease, congestive heart failure, and renal failure, and thus results in considerable morbidity and mortality each year.8
Contaminant Exposure and Hypertension Risk
Vietnam-Era Army Chemical Corps
The US Army Chemical Corps (ACC) Vietnam-Era Veterans Health Study (2012-2013) recorded the long-term health burdens imposed by Agent Orange exposure and Vietnam War service.9,10 This cross-sectional study reexamined a subset of 5,609 Vietnam-era ACC veterans for an association of self-reported, physician-diagnosed hypertension (≥ 140/90 mm Hg) and herbicide spraying history confirmed with serum TCDD levels. The 22 Army units that made up the ACC were in charge of spreading Agent Orange and other defoliants on opposition camps between 1965 and 1973. The herbicide was dispersed aerially and on the ground. The ACC was also responsible for dispensing napalm, tear gas, and other chemicals.
A previous phone survey found an association of self-reported hypertension and herbicide spraying in ACC veterans with associated Vietnam service and herbicide spraying history, verified with serum TCDD levels (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.00-1.58).9 Median age of ACC veterans with Vietnam War service at the time of the survey was 53 years.
The 2012-2013 study assessed respondents with a record of their serum TCDD measurements from the time of the survey.10 Most of the respondents were aged in their 60s. The stated purpose of the health study was to examine the association of Vietnam veterans’ occupational herbicide exposure and hypertension risk, rather than isolate a certain responsible chemical, though serum TCDD levels were used to confirm spraying history. After adjustments for age, tobacco use, alcohol use, race, and BMI but not salt intake, family history of hypertension, psychiatric health, or diabetes mellitus (DM) comorbidity, the OR of self-reported, physician-diagnosed hypertension was 1.74 (95% CI, 1.44-2.11) for sprayers and 1.26 (95% CI, 1.05-1.53) for Vietnam veterans.10
Vietnam War Veterans From Korea
Soldiers of the Republic of Korea (ROK) who fought in the Vietnam War also were exposed to Agent Orange and other defoliants and herbicides. In 2013, Yi and colleagues contacted 187,897 ROK Vietnam veterans to analyze their Agent Orange exposure and self-reported diseases decades after the war.11 By mail, the researchers administered a questionnaire of perceived Agent Orange exposure (eg, spraying, handling spray equipment, having contact with COIs). The Korean veterans were classified by military assignment and by their proximity to areas sprayed with Agent Orange, according to the military records of 3 US combat units: Capital Division, 9th Division, and Marine Second Brigade. The ROK veterans in those units presumably would have similar levels of Agent Orange exposure.
The questionnaire response rate was 69%. The 114,562 respondents were divided into groups based on self-perceived exposure (no, low, moderate, high) and qualitative exposure level, derived from service history (battalion/company, division/brigade). After adjusting for BMI, smoking, alcohol use, physical activity, use of nonoccupational herbicides, education, income, and military rank, Yi and colleagues found a statistically significant association of hypertension and self-reported perceived Agent Orange exposure (P < .001) and a statistically significant association of hypertension and exposure in the division/brigade group with the highest exposure level (P < .001).11 The highest ORs were found for high- vs low-exposure and moderate- vs low-exposure subsets in self-reported perceived Agent Orange levels: 1.60 (95% CI, 1.56-1.65) and 1.70 (95% CI, 1.64-1.77), respectively. However, adjusted ORs in proximity-based exposure for all groups were > 1.03.
Inuits in Canada and Greenland
To study total PCBs, non-dioxin-like PCBs, OC pesticides, and their metabolites in plasma, public health researchers Valera and colleagues focused on the Inuit town of Nunavik (in Canada), where there is contamination from foods like fish, a mainstay of the Inuit diet.5 A health survey was sent to 400 households randomly selected from 1,378 households in 14 villages. Data were collected between September and December 1992. In total, 518 people between ages 18 and 74 years agreed to undergo a physical examination, and 492 agreed to have blood drawn. Laboratories measured serum PCB congeners and 13 chlorinated pesticides or their metabolites. Blood pressure (BP) was measured 3 times, and the last 2 measurements averaged. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg.
Of the 518 participants, 315 (134 men, 181 women) had complete BP, serum POP levels, and confounding variables recorded, and these were subsequently analyzed. Mean age was 32.7 years. Polychlorinated biphenyls congeners 105 and 118 were higher in women than in men; no other congeners were quantitatively different. Associations between POP levels and hypertension were analyzed with multiple logistic regression modeling, with adjustments for age, sex, fasting blood glucose, waist circumference, smoking, alcohol use, and physical activity, as well as the common contaminants lead, mercury, and omega-3 polyunsaturated fatty acids (n-3 PUFA).The researchers adjusted for n-3 PUFA because of the posited BP-lowering effects. Inuits consume large amounts of the polyunsaturated fatty acids DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid).5
Using congeners found in at least 70% of the total samples, the study authors found a statistically significant association between certain PCBs, both dioxin-like (DL-PCBs) and non-dioxin-like (NDL-PCBs), and increased risk of hypertension. Congeners 101, 105 (DL-PCB), 138, and 187, as well as p,p'-DDE, were also significantly associated with a higher risk of hypertension. Congener 99 was associated with increased SBP, and congener 118 (DL-PCB) was associated with increased SBP and DBP. Some congeners, such as the OC pesticides, p,p'-DDT, β-hexachlorocyclohexane, and oxychlordane, were inversely associated with hypertension.
In 2012, Valera and colleagues conducted a similar study of Greenland Inuits who also consume marine mammals and fish and present with high POP levels.6 Despite correcting for n-3 PUFA, they found no significant association involving DL-PCBs, NDL-PCBs, or OC pesticides.
Japanese Background Exposures
Nakamoto and colleagues conducted a cross-sectional study of 2,266 Japanese women and men who had been exposed to background (vs occupational or wartime) levels of dioxins, including PCDDs, PCDFs, and DL-PCBs.12 The dioxins likely originated from combustion of chlorinated materials and older manufactured electronics components. The study participants had lived in urban areas or in farming or fishing villages for at least 10 consecutive years and had no concomitant occupational exposure to dioxins. Mean (SD) age was 43.5 (13.6) years for the men and 45.3 (14.0) years for the women. Participants volunteered their disease histories, which included physician-diagnosed hypertension (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg). Logistic regression analyses were adjusted for BMI, sex, age, regional residential area, smoking, alcohol use, and survey year. In fishing regions, PCDDs, PCDFs, and DL-PCBs were significantly higher than in the other regions. Of the 2,266 participants, 647 reported physician-diagnosed hypertension. Dividing the toxic equivalents of serum COI levels into quartiles of concentration, Nakamoto and colleagues found a statistically significant association of hypertension and increased toxic equivalent levels of PCDDs, PCDFs, DL-PCBs, and total dioxins.
Italian Male Steelworkers
In a 2016 retrospective cohort study, Cappelletti and colleagues assessed the health burden of workers at a steel recycling plant in Trento, Italy. The plant, which had been using an electric arc furnace without a coke oven, had been exposing workers to dust containing PCBs, PCDDs, PCDFs, and other metals.13 Each hour, roughly 2 to 5 kg of dust was being released inside the plant (diffuse emissions), and exposure extended to a 2-km radius around the plant. A cohort of 331 plant workers, identified and assessed through company records, had been exposed to diffuse emissions for at least 1 year between 1979 and 2009. This group was compared with a control group of 32 office workers from that company, as identified by company records. The authors found a risk ratio (RR) of 2.23 in cases of noncomplicated hypertension and an RR of 2.01 in cases of complicated hypertension, defined as hypertension with organ damage.
Elderly in Sweden
In a study of 1,016 Swedish men and women who were aged 70 years or older and were living in Uppsala, Sweden, Lind and colleagues calculated average supine BP from 3 sphygmomanometer measurements after 30 minutes of rest.14 The researchers used high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) to measure the serum levels of a set of 23 POPs—16 PCB congeners, 5 OC pesticides, 1 brominated biphenyl ether congener, and octachloro-p-dibenzodioxin—and lipid-normalized the values. They used logistic regression to assess POP levels and prevalent hypertension (BP ≥ 140/90 mm Hg or use of antihypertensives), adjusting for sex, BMI, smoking status, exercise, and education. Among the COIs with the highest circulating lipid-normalized POP levels were PCB congeners 180, 138, and 170 and DDE. There was no clear relationship between toxic equivalents and hypertension; after multivariate adjustments, only DDE showed a statistically significant OR: 1.25 (95% CI, 1.07-1.47).
Organic Pollutants and Hypertension
Using National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2002, Ha and colleagues conducted a cross-sectional study of a 524-adult subset of patients who were exposed to background levels of POPs and had newly diagnosed hypertension (≥ 140/90 mm Hg).15 In the NHANES study, the CDC collected standardized patient history information, physical examination findings, and venous blood sample results. Recorded BP data points were the averages of 3 separate SBP and DBP readings from a sphygmomanometer, as recommended by the AHA. The NHANES study recorded POPs with HRGC/HRMS.
Ha and colleagues selected 12 POPs, and standardized the COI concentrations against lipid concentration. The lipid-standardized POP concentrations used were at a higher level of detection and found in at least 60% of the study patients. The researchers used a logistic regression model to calculate multivariate-adjusted OR separately in men and women, adjusting for race/ethnicity, smoking/alcohol use, physical activity, BMI, cotinine level, and income level. Among the 56 men and 67 women with newly diagnosed prevalent hypertension, PCDD levels in women were positively associated with hypertension but not correlated with higher or lower toxic equivalency factors. Dioxin and NDL-PCBs were positively associated with hypertension in men but negatively in women. Ha and colleagues postulated that this approach of studying a US population subset of patients with background exposure to POPs, instead of groups with high concentrations of exposure (eg, Vietnam War veterans or those exposed occupationally or in industrial accidents), provides an alternative observable effect of long-term, low-dose exposure of a blend of POPs.15
Discussion
In vivo and in vitro studies have found that dioxins induce a subset of 35 genes, including microsomal P450 enzymes, kinases and phosphates, and DNA repair proteins. A microarray profile of cardiovascular murine tissue and cultured vascular smooth muscle cells exposed to TCDD found known dioxin-inducible genes Cyp1b1, a phase 1 drug metabolism enzyme, and Aldh3A1, another drug metabolism gene up-regulated, among lectin-related natural killer cell receptor, insulin-like growth factor binding protein, and cyclin G2.16
Dioxins bind avidly to the aryl hydrocarbon receptor (AhR), a cytosolic transcription factor that also interacts with other xenobiotic compounds with varying affinities. TCDD is one of the most potent ligands for AhR, and other DL compounds have a lower binding affinity. AhR dimerizes in the nucleus with the AhR nuclear translocator and then binds genomic dioxin response elements and induces the expression of cytochrome P450 genes, such as CYP1A1.17
The AhRs are highly expressed in the vascular endothelium.17 Agbor and colleagues found that mice with endothelial AhR knockouts showed decreased baseline SBP and DBP.18 When challenged with angiotensin II, a potent vasoconstrictor, AhR-/- mice failed to show an increase in DBP. AhR-/- exhibited reduced ex vivo aortic contraction in the presence of angiotensin II in aortas with perivascular adipose tissue. Notably, compared with wild-type mice, AhR-/- mice had reduced renin-angiotensinsystem gene expression in the visceral adipose, linking the AhR receptor with the endogenous renin-angiotensin-aldosterone system (RAAS).
Early studies have shown that mice lacking AhR do not demonstrate TCDD toxicity.20 More recently, Kopf and colleagues found that TCDD exposure in mice led to increased BP and cardiac hypertrophy, possibly linked to increased superoxide production in the vasculature.21 When exposed to TCDD, mice showed enhanced CYP1A1 mRNA expression in the left ventricle, kidney, and aorta by day 35 and increased CYP1B1 mRNA expression in the left ventricle after 60 days. Within the first week of TCDD exposure, the mean arterial pressure for the exposure group was statistically significantly increased, showing a trend of peaks and plateaus. Mice exposed to TCDD also showed left ventricular concentric hypertrophy, which is typical of systemic hypertension.8,21 Kerley-Hamilton and colleagues found that AhR ligand activation increased atherosclerosis.22
Most hypertension is idiopathic. Research into the downstream effects of AhR suggests it induces vascular oxidative stress and increases atherosclerosis.22 It is unclear whether this is an initiating or synergistic factor in the development of hypertension. The study results described here indicate that dioxins initiate BP changes through the endothelial AhR receptor, but this mechanism has been proved only in an animal model. Ongoing studies are needed to examine the molecular changes in humans. Clinicians can be advised that dioxin exposure, rather than being an initiating factor, would at most contribute to an accumulating series of assaults, including genetics, lifestyle, and environmental factors, and that these assaults progress to hypertension only after passing a threshold.23 Moreover, many of the studies described here categorized hypertension under the guideline of 140/90 mm Hg. Future studies may use the newer guideline, which will affect their results.
Conclusion
Studies have shown an association between dioxins and endocrine disruption, reproductive and developmental problems, and certain cancers.3,24 The Seveso Women’s Health Study of an industrial accident in Italy linked dioxins to an incidence of DM, obesity, or metabolic syndrome.25 By contrast, evidence of a link between dioxins and hypertension has been limited and inconsistent. Seven of the 8 studies reviewed in this study found moderate evidence of association in patients with at least 1 chemical congener and a certain subset of the study population (Table).
The Vietnam-Era Veterans Health Study found a higher OR of developing hypertension in herbicide sprayers than in its control group. Korean Vietnam War veterans stratified by either self-reported risk or military assignment also had significant associations. For male steelworkers in Italy, occupational exposure had a moderately higher RR in the exposure cohort. In the NHANES study, background levels of POPs were positively associated, but only in men. A nonoccupational study in urban and rural areas of Japan found a significant association between dioxins and hypertension. A nonoccupational study of elderly Swedes found a significant association with only 1 chemical congener. A study of Inuits in Greenland found no significant associations, but a study of Inuits in Canada did yield an association.Recent studies maintain the 2012 veterans update regarding a limited but suggestive association of dioxin and hypertension.4 Despite having high power because of the number of exposed patients, these observational studies can posit only an associative relationship, not a causal one. These studies also are limited by their categorization of dioxin exposure levels—ranging from perceived exposure to proximity and direct serum dioxin measurement. Moreover, chemical levels are measured an inconsistent number of years after exposure, and therefore, as dioxins are primarily metabolized by CYP genes, different metabolic rates could account for different susceptibility to health effects.2
In vivo animal studies could better characterize the effect of time point of exposure and effects on hypertension. Studies could also examine the synergistic effects of dioxins and other toxins, or smoking or alcohol use, on hypertension. New clinical guidelines for hypertension will have an impact on studies. Overall, clinicians who treat patients with known exposure to dioxins can suggest with moderate confidence that it is likely not a primary reason for the development of hypertension. At most, dioxin exposure is a contributing factor in the development of hypertension, with lifestyle, smoking, diet, and genetics playing more compelling roles.
Persistent organic pollutants (POPs), endocrine-disrupting, lipophilic chemicals that concentrate in adipose tissue, increasingly are being studied for a wide range of health effects.1 Persistent organic pollutants include bisphenol A, phthalates, dioxins, hexachlorobenzene, dichlorodiphenyltrichloroethane (DDT), polybrominated diphenyl ethers, and polychlorinated biphenyls (PCBs). Chlorinated dibenzo-p-dioxins are known as polychlorinated dibenzodioxins (PCDDs), or simply dioxins. Categorization of this group of chemicals is based on the structural chlorinated constituents. Of the 75 congener molecules, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic, and this dioxin, given its more serious health implications, has been studied the most.1,2
Because it was a contaminant in the herbicide Agent Orange, the main defoliant used by the US military in southern Vietnam during the Vietnam War, TCDD is of primary interest. Agent Orange consists of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in equal parts. Like other dioxins, TCDD is lipophilic and retained in adipose tissue.1,3 Contemporaneous sources include occupational and residential exposure from pulp and paper mills, metallurgy, incinerators, industrial waste, fossil fuel combustion, and industrial accidents and poisonings.1-4
Another main class of POPs, polychlorinated benzenes, includes 209 synthetic PCB congener chemicals, a subset of which is referred to as dioxin-like PCBs.1 Organochlorine (OC) pesticides and PCBs were once manufactured as lubricants and coolants for electronics but are now banned; nevertheless, they remain concentrated in fish and mammals and persist in the food chain.3,5,6 These chemicals of interest (COIs) are graded for toxicity based on toxic equivalency factors relative to TCDDs in a 2005 World Health Organization assessment.3
Polychlorinated dibenzofurans (PCDFs), TCDD, PCBs, PCDDs, and other environmental toxins are being studied as possible contributing factors in the development of hypertension. The authors review the results of several recent studies on COI exposure and hypertension.
In 2017, the American College of Cardiology and the American Heart Association lowered the threshold for hypertension to systolic blood pressure (SBP) > 130 mm Hg and diastolic blood pressure (DBP) > 80 mm Hg.7 This new guideline would categorize 46% of the US population as having hypertension, compared with 32% under the former cutoff of 140/90 mm Hg.7 Modifiable factors (eg, diet, body mass index [BMI], smoking, alcohol, physical activity) and nonmodifiable factors (eg, age, family history, sex, race/ethnicity) have a role in the pathophysiology of hypertension. Between 90% and 95% of hypertension is considered primary. Hypertension increases the risk of developing ischemic heart disease, atherosclerosis, aortic dissection, small blood vessel disease, congestive heart failure, and renal failure, and thus results in considerable morbidity and mortality each year.8
Contaminant Exposure and Hypertension Risk
Vietnam-Era Army Chemical Corps
The US Army Chemical Corps (ACC) Vietnam-Era Veterans Health Study (2012-2013) recorded the long-term health burdens imposed by Agent Orange exposure and Vietnam War service.9,10 This cross-sectional study reexamined a subset of 5,609 Vietnam-era ACC veterans for an association of self-reported, physician-diagnosed hypertension (≥ 140/90 mm Hg) and herbicide spraying history confirmed with serum TCDD levels. The 22 Army units that made up the ACC were in charge of spreading Agent Orange and other defoliants on opposition camps between 1965 and 1973. The herbicide was dispersed aerially and on the ground. The ACC was also responsible for dispensing napalm, tear gas, and other chemicals.
A previous phone survey found an association of self-reported hypertension and herbicide spraying in ACC veterans with associated Vietnam service and herbicide spraying history, verified with serum TCDD levels (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.00-1.58).9 Median age of ACC veterans with Vietnam War service at the time of the survey was 53 years.
The 2012-2013 study assessed respondents with a record of their serum TCDD measurements from the time of the survey.10 Most of the respondents were aged in their 60s. The stated purpose of the health study was to examine the association of Vietnam veterans’ occupational herbicide exposure and hypertension risk, rather than isolate a certain responsible chemical, though serum TCDD levels were used to confirm spraying history. After adjustments for age, tobacco use, alcohol use, race, and BMI but not salt intake, family history of hypertension, psychiatric health, or diabetes mellitus (DM) comorbidity, the OR of self-reported, physician-diagnosed hypertension was 1.74 (95% CI, 1.44-2.11) for sprayers and 1.26 (95% CI, 1.05-1.53) for Vietnam veterans.10
Vietnam War Veterans From Korea
Soldiers of the Republic of Korea (ROK) who fought in the Vietnam War also were exposed to Agent Orange and other defoliants and herbicides. In 2013, Yi and colleagues contacted 187,897 ROK Vietnam veterans to analyze their Agent Orange exposure and self-reported diseases decades after the war.11 By mail, the researchers administered a questionnaire of perceived Agent Orange exposure (eg, spraying, handling spray equipment, having contact with COIs). The Korean veterans were classified by military assignment and by their proximity to areas sprayed with Agent Orange, according to the military records of 3 US combat units: Capital Division, 9th Division, and Marine Second Brigade. The ROK veterans in those units presumably would have similar levels of Agent Orange exposure.
The questionnaire response rate was 69%. The 114,562 respondents were divided into groups based on self-perceived exposure (no, low, moderate, high) and qualitative exposure level, derived from service history (battalion/company, division/brigade). After adjusting for BMI, smoking, alcohol use, physical activity, use of nonoccupational herbicides, education, income, and military rank, Yi and colleagues found a statistically significant association of hypertension and self-reported perceived Agent Orange exposure (P < .001) and a statistically significant association of hypertension and exposure in the division/brigade group with the highest exposure level (P < .001).11 The highest ORs were found for high- vs low-exposure and moderate- vs low-exposure subsets in self-reported perceived Agent Orange levels: 1.60 (95% CI, 1.56-1.65) and 1.70 (95% CI, 1.64-1.77), respectively. However, adjusted ORs in proximity-based exposure for all groups were > 1.03.
Inuits in Canada and Greenland
To study total PCBs, non-dioxin-like PCBs, OC pesticides, and their metabolites in plasma, public health researchers Valera and colleagues focused on the Inuit town of Nunavik (in Canada), where there is contamination from foods like fish, a mainstay of the Inuit diet.5 A health survey was sent to 400 households randomly selected from 1,378 households in 14 villages. Data were collected between September and December 1992. In total, 518 people between ages 18 and 74 years agreed to undergo a physical examination, and 492 agreed to have blood drawn. Laboratories measured serum PCB congeners and 13 chlorinated pesticides or their metabolites. Blood pressure (BP) was measured 3 times, and the last 2 measurements averaged. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg.
Of the 518 participants, 315 (134 men, 181 women) had complete BP, serum POP levels, and confounding variables recorded, and these were subsequently analyzed. Mean age was 32.7 years. Polychlorinated biphenyls congeners 105 and 118 were higher in women than in men; no other congeners were quantitatively different. Associations between POP levels and hypertension were analyzed with multiple logistic regression modeling, with adjustments for age, sex, fasting blood glucose, waist circumference, smoking, alcohol use, and physical activity, as well as the common contaminants lead, mercury, and omega-3 polyunsaturated fatty acids (n-3 PUFA).The researchers adjusted for n-3 PUFA because of the posited BP-lowering effects. Inuits consume large amounts of the polyunsaturated fatty acids DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid).5
Using congeners found in at least 70% of the total samples, the study authors found a statistically significant association between certain PCBs, both dioxin-like (DL-PCBs) and non-dioxin-like (NDL-PCBs), and increased risk of hypertension. Congeners 101, 105 (DL-PCB), 138, and 187, as well as p,p'-DDE, were also significantly associated with a higher risk of hypertension. Congener 99 was associated with increased SBP, and congener 118 (DL-PCB) was associated with increased SBP and DBP. Some congeners, such as the OC pesticides, p,p'-DDT, β-hexachlorocyclohexane, and oxychlordane, were inversely associated with hypertension.
In 2012, Valera and colleagues conducted a similar study of Greenland Inuits who also consume marine mammals and fish and present with high POP levels.6 Despite correcting for n-3 PUFA, they found no significant association involving DL-PCBs, NDL-PCBs, or OC pesticides.
Japanese Background Exposures
Nakamoto and colleagues conducted a cross-sectional study of 2,266 Japanese women and men who had been exposed to background (vs occupational or wartime) levels of dioxins, including PCDDs, PCDFs, and DL-PCBs.12 The dioxins likely originated from combustion of chlorinated materials and older manufactured electronics components. The study participants had lived in urban areas or in farming or fishing villages for at least 10 consecutive years and had no concomitant occupational exposure to dioxins. Mean (SD) age was 43.5 (13.6) years for the men and 45.3 (14.0) years for the women. Participants volunteered their disease histories, which included physician-diagnosed hypertension (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg). Logistic regression analyses were adjusted for BMI, sex, age, regional residential area, smoking, alcohol use, and survey year. In fishing regions, PCDDs, PCDFs, and DL-PCBs were significantly higher than in the other regions. Of the 2,266 participants, 647 reported physician-diagnosed hypertension. Dividing the toxic equivalents of serum COI levels into quartiles of concentration, Nakamoto and colleagues found a statistically significant association of hypertension and increased toxic equivalent levels of PCDDs, PCDFs, DL-PCBs, and total dioxins.
Italian Male Steelworkers
In a 2016 retrospective cohort study, Cappelletti and colleagues assessed the health burden of workers at a steel recycling plant in Trento, Italy. The plant, which had been using an electric arc furnace without a coke oven, had been exposing workers to dust containing PCBs, PCDDs, PCDFs, and other metals.13 Each hour, roughly 2 to 5 kg of dust was being released inside the plant (diffuse emissions), and exposure extended to a 2-km radius around the plant. A cohort of 331 plant workers, identified and assessed through company records, had been exposed to diffuse emissions for at least 1 year between 1979 and 2009. This group was compared with a control group of 32 office workers from that company, as identified by company records. The authors found a risk ratio (RR) of 2.23 in cases of noncomplicated hypertension and an RR of 2.01 in cases of complicated hypertension, defined as hypertension with organ damage.
Elderly in Sweden
In a study of 1,016 Swedish men and women who were aged 70 years or older and were living in Uppsala, Sweden, Lind and colleagues calculated average supine BP from 3 sphygmomanometer measurements after 30 minutes of rest.14 The researchers used high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) to measure the serum levels of a set of 23 POPs—16 PCB congeners, 5 OC pesticides, 1 brominated biphenyl ether congener, and octachloro-p-dibenzodioxin—and lipid-normalized the values. They used logistic regression to assess POP levels and prevalent hypertension (BP ≥ 140/90 mm Hg or use of antihypertensives), adjusting for sex, BMI, smoking status, exercise, and education. Among the COIs with the highest circulating lipid-normalized POP levels were PCB congeners 180, 138, and 170 and DDE. There was no clear relationship between toxic equivalents and hypertension; after multivariate adjustments, only DDE showed a statistically significant OR: 1.25 (95% CI, 1.07-1.47).
Organic Pollutants and Hypertension
Using National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2002, Ha and colleagues conducted a cross-sectional study of a 524-adult subset of patients who were exposed to background levels of POPs and had newly diagnosed hypertension (≥ 140/90 mm Hg).15 In the NHANES study, the CDC collected standardized patient history information, physical examination findings, and venous blood sample results. Recorded BP data points were the averages of 3 separate SBP and DBP readings from a sphygmomanometer, as recommended by the AHA. The NHANES study recorded POPs with HRGC/HRMS.
Ha and colleagues selected 12 POPs, and standardized the COI concentrations against lipid concentration. The lipid-standardized POP concentrations used were at a higher level of detection and found in at least 60% of the study patients. The researchers used a logistic regression model to calculate multivariate-adjusted OR separately in men and women, adjusting for race/ethnicity, smoking/alcohol use, physical activity, BMI, cotinine level, and income level. Among the 56 men and 67 women with newly diagnosed prevalent hypertension, PCDD levels in women were positively associated with hypertension but not correlated with higher or lower toxic equivalency factors. Dioxin and NDL-PCBs were positively associated with hypertension in men but negatively in women. Ha and colleagues postulated that this approach of studying a US population subset of patients with background exposure to POPs, instead of groups with high concentrations of exposure (eg, Vietnam War veterans or those exposed occupationally or in industrial accidents), provides an alternative observable effect of long-term, low-dose exposure of a blend of POPs.15
Discussion
In vivo and in vitro studies have found that dioxins induce a subset of 35 genes, including microsomal P450 enzymes, kinases and phosphates, and DNA repair proteins. A microarray profile of cardiovascular murine tissue and cultured vascular smooth muscle cells exposed to TCDD found known dioxin-inducible genes Cyp1b1, a phase 1 drug metabolism enzyme, and Aldh3A1, another drug metabolism gene up-regulated, among lectin-related natural killer cell receptor, insulin-like growth factor binding protein, and cyclin G2.16
Dioxins bind avidly to the aryl hydrocarbon receptor (AhR), a cytosolic transcription factor that also interacts with other xenobiotic compounds with varying affinities. TCDD is one of the most potent ligands for AhR, and other DL compounds have a lower binding affinity. AhR dimerizes in the nucleus with the AhR nuclear translocator and then binds genomic dioxin response elements and induces the expression of cytochrome P450 genes, such as CYP1A1.17
The AhRs are highly expressed in the vascular endothelium.17 Agbor and colleagues found that mice with endothelial AhR knockouts showed decreased baseline SBP and DBP.18 When challenged with angiotensin II, a potent vasoconstrictor, AhR-/- mice failed to show an increase in DBP. AhR-/- exhibited reduced ex vivo aortic contraction in the presence of angiotensin II in aortas with perivascular adipose tissue. Notably, compared with wild-type mice, AhR-/- mice had reduced renin-angiotensinsystem gene expression in the visceral adipose, linking the AhR receptor with the endogenous renin-angiotensin-aldosterone system (RAAS).
Early studies have shown that mice lacking AhR do not demonstrate TCDD toxicity.20 More recently, Kopf and colleagues found that TCDD exposure in mice led to increased BP and cardiac hypertrophy, possibly linked to increased superoxide production in the vasculature.21 When exposed to TCDD, mice showed enhanced CYP1A1 mRNA expression in the left ventricle, kidney, and aorta by day 35 and increased CYP1B1 mRNA expression in the left ventricle after 60 days. Within the first week of TCDD exposure, the mean arterial pressure for the exposure group was statistically significantly increased, showing a trend of peaks and plateaus. Mice exposed to TCDD also showed left ventricular concentric hypertrophy, which is typical of systemic hypertension.8,21 Kerley-Hamilton and colleagues found that AhR ligand activation increased atherosclerosis.22
Most hypertension is idiopathic. Research into the downstream effects of AhR suggests it induces vascular oxidative stress and increases atherosclerosis.22 It is unclear whether this is an initiating or synergistic factor in the development of hypertension. The study results described here indicate that dioxins initiate BP changes through the endothelial AhR receptor, but this mechanism has been proved only in an animal model. Ongoing studies are needed to examine the molecular changes in humans. Clinicians can be advised that dioxin exposure, rather than being an initiating factor, would at most contribute to an accumulating series of assaults, including genetics, lifestyle, and environmental factors, and that these assaults progress to hypertension only after passing a threshold.23 Moreover, many of the studies described here categorized hypertension under the guideline of 140/90 mm Hg. Future studies may use the newer guideline, which will affect their results.
Conclusion
Studies have shown an association between dioxins and endocrine disruption, reproductive and developmental problems, and certain cancers.3,24 The Seveso Women’s Health Study of an industrial accident in Italy linked dioxins to an incidence of DM, obesity, or metabolic syndrome.25 By contrast, evidence of a link between dioxins and hypertension has been limited and inconsistent. Seven of the 8 studies reviewed in this study found moderate evidence of association in patients with at least 1 chemical congener and a certain subset of the study population (Table).
The Vietnam-Era Veterans Health Study found a higher OR of developing hypertension in herbicide sprayers than in its control group. Korean Vietnam War veterans stratified by either self-reported risk or military assignment also had significant associations. For male steelworkers in Italy, occupational exposure had a moderately higher RR in the exposure cohort. In the NHANES study, background levels of POPs were positively associated, but only in men. A nonoccupational study in urban and rural areas of Japan found a significant association between dioxins and hypertension. A nonoccupational study of elderly Swedes found a significant association with only 1 chemical congener. A study of Inuits in Greenland found no significant associations, but a study of Inuits in Canada did yield an association.Recent studies maintain the 2012 veterans update regarding a limited but suggestive association of dioxin and hypertension.4 Despite having high power because of the number of exposed patients, these observational studies can posit only an associative relationship, not a causal one. These studies also are limited by their categorization of dioxin exposure levels—ranging from perceived exposure to proximity and direct serum dioxin measurement. Moreover, chemical levels are measured an inconsistent number of years after exposure, and therefore, as dioxins are primarily metabolized by CYP genes, different metabolic rates could account for different susceptibility to health effects.2
In vivo animal studies could better characterize the effect of time point of exposure and effects on hypertension. Studies could also examine the synergistic effects of dioxins and other toxins, or smoking or alcohol use, on hypertension. New clinical guidelines for hypertension will have an impact on studies. Overall, clinicians who treat patients with known exposure to dioxins can suggest with moderate confidence that it is likely not a primary reason for the development of hypertension. At most, dioxin exposure is a contributing factor in the development of hypertension, with lifestyle, smoking, diet, and genetics playing more compelling roles.
1. Van den Berg M, Birnbaum L, Bosveld AT, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106(12):775-792.
2. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Koplan JP. Toxicological profile for chlorinated dibenzo-p-dioxins. https://www.atsdr.cdc .gov/toxprofiles/tp104.pdf. Published December 1998. Accessed April 3, 2018.
3. Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223-241.
4. Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Ninth Biennial Update); Board of the Health of Select Populations, Institute of Medicine. Veterans and Agent Orange: Update 2012. Washington, DC: National Academies Press; 2014.
5. Valera B, Ayotte P, Poirier P, Dewailly E. Associations between plasma persistent organic pollutant levels and blood pressure in Inuit adults from Nunavik. Environ Int. 2013;59:282-289.
6. Valera B, Jørgensen ME, Jeppesen C, Bjerregaard P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. 2013;122:65-73.
7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;Nov 13:pii:HYP.0000000000000066. [Epub ahead of print.]
8. Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier; 2014.
9. Kang HK, Dalager NA, Needham LL, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006;49(11):875-884.
10. Cypel YS, Kress AM, Eber SM, Schneiderman AI, Davey VJ. Herbicide exposure, Vietnam service, and hypertension risk in Army Chemical Corps veterans. J Occup Environ Med. 2016;58(11):1127-1136.
11. Yi SW, Ohrr H, Hong JS, Yi JJ. Agent Orange exposure and prevalence of self-reported diseases in Korean Vietnam veterans. J Prev Med Public Health. 2013;46(5):213-225.
12. Nakamoto M, Arisawa K, Uemura H, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health. 2013;86(8):849-859.
13. Cappelletti R, Ceppi M, Claudatus J, Gennaro V. Health status of male steel workers at an electric arc furnace (EAF) in Trentino, Italy. J Occup Med Toxicol. 2016;11:7.
14. Lind PM, Penell J, Salihovic S, van Bavel B, Lind L. Circulating levels of p,p’-DDE are related to prevalent hypertension in the elderly. Environ Res. 2014;129:27-31.
15. Ha MH, Lee DH, Son HK, Park SK, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999–2002. J Hum Hypertens. 2009;23(4):274-286.
16. Puga A, Sartor MA, Huang M, et al. Gene expression profiles of mouse aorta and cultured vascular smooth muscle cells differ widely, yet show common responses to dioxin exposure. Cardiovasc Toxicol. 2004;4(4):385-404.
17. Swanson HI, Bradfield CA. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993;3(5):213-230.
18. Agbor LN, Elased KM, Walker MK. Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness. Biochem Pharmacol. 2011;82(5):514-523.
19. Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338(1):311-317.
20. Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173-179.
21. Kopf PG, Scott JA, Agbor LN, et al. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117(2):537-546.
22. Kerley-Hamilton JS, Trask HW, Ridley CJ, et al. Inherent and benzo[a]pyrene-induced differential aryl hydrocarbon receptor signaling greatly affects life span, atherosclerosis, cardiac gene expression, and body and heart growth in mice. Toxicol Sci. 2012;126(2):391-404.
23. Narkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Pressure. 2005;14(2):69-71.
24. Kogevinas M. Human health effects of dioxins: cancer, reproductive and endocrine system effects. Hum Reprod Update. 2001;7(3):331-339.
25. Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso Women’s Health Study. Environ Health Perspect. 2013;121(8):906-911.
1. Van den Berg M, Birnbaum L, Bosveld AT, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106(12):775-792.
2. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Koplan JP. Toxicological profile for chlorinated dibenzo-p-dioxins. https://www.atsdr.cdc .gov/toxprofiles/tp104.pdf. Published December 1998. Accessed April 3, 2018.
3. Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223-241.
4. Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Ninth Biennial Update); Board of the Health of Select Populations, Institute of Medicine. Veterans and Agent Orange: Update 2012. Washington, DC: National Academies Press; 2014.
5. Valera B, Ayotte P, Poirier P, Dewailly E. Associations between plasma persistent organic pollutant levels and blood pressure in Inuit adults from Nunavik. Environ Int. 2013;59:282-289.
6. Valera B, Jørgensen ME, Jeppesen C, Bjerregaard P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. 2013;122:65-73.
7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;Nov 13:pii:HYP.0000000000000066. [Epub ahead of print.]
8. Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier; 2014.
9. Kang HK, Dalager NA, Needham LL, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006;49(11):875-884.
10. Cypel YS, Kress AM, Eber SM, Schneiderman AI, Davey VJ. Herbicide exposure, Vietnam service, and hypertension risk in Army Chemical Corps veterans. J Occup Environ Med. 2016;58(11):1127-1136.
11. Yi SW, Ohrr H, Hong JS, Yi JJ. Agent Orange exposure and prevalence of self-reported diseases in Korean Vietnam veterans. J Prev Med Public Health. 2013;46(5):213-225.
12. Nakamoto M, Arisawa K, Uemura H, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health. 2013;86(8):849-859.
13. Cappelletti R, Ceppi M, Claudatus J, Gennaro V. Health status of male steel workers at an electric arc furnace (EAF) in Trentino, Italy. J Occup Med Toxicol. 2016;11:7.
14. Lind PM, Penell J, Salihovic S, van Bavel B, Lind L. Circulating levels of p,p’-DDE are related to prevalent hypertension in the elderly. Environ Res. 2014;129:27-31.
15. Ha MH, Lee DH, Son HK, Park SK, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999–2002. J Hum Hypertens. 2009;23(4):274-286.
16. Puga A, Sartor MA, Huang M, et al. Gene expression profiles of mouse aorta and cultured vascular smooth muscle cells differ widely, yet show common responses to dioxin exposure. Cardiovasc Toxicol. 2004;4(4):385-404.
17. Swanson HI, Bradfield CA. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993;3(5):213-230.
18. Agbor LN, Elased KM, Walker MK. Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness. Biochem Pharmacol. 2011;82(5):514-523.
19. Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338(1):311-317.
20. Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173-179.
21. Kopf PG, Scott JA, Agbor LN, et al. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117(2):537-546.
22. Kerley-Hamilton JS, Trask HW, Ridley CJ, et al. Inherent and benzo[a]pyrene-induced differential aryl hydrocarbon receptor signaling greatly affects life span, atherosclerosis, cardiac gene expression, and body and heart growth in mice. Toxicol Sci. 2012;126(2):391-404.
23. Narkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Pressure. 2005;14(2):69-71.
24. Kogevinas M. Human health effects of dioxins: cancer, reproductive and endocrine system effects. Hum Reprod Update. 2001;7(3):331-339.
25. Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso Women’s Health Study. Environ Health Perspect. 2013;121(8):906-911.