User login
From the Oregon Health and Science University, Portland, OR.
Abstract
- Objective: To describe the current approach to diagnosis and treatment of aplastic anemia.
- Methods: Review of the literature.
- Results: Aplastic anemia can be acquired or associated with an inherited marrow failure syndrome (IMFS), and the treatment and prognosis vary dramatically between these 2 etiologies. Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to life-threatening neutropenic infections or bleeding. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia.
- Conclusion: Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marro
w transplant program to evaluate for early transplantation is the new standard of care for aplastic anemia.
Keywords: inherited marrow failure syndrome; Fanconi anemia; immunosuppression; transplant; stem cell.
Aplastic anemia is a clinical and pathological entity of bone marrow failure that causes progressive loss of hematopoietic progenitor stem cells (HPSC), resulting in pancytopenia.1 Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to having life-threatening neutropenic infections or bleeding. Aplastic anemia results from either inherited or acquired causes, and the pathophysiology and treatment approach vary significantly between these 2 causes. Therefore, recognition of inherited marrow failure diseases, such as Fanconi anemia and telomere biology disorders, is critical to establishing the management plan.
Epidemiology
Aplastic anemia is a rare disorder, with an incidence of approximately 1.5 to 7 cases per million individuals per year.2,3 A recent Scandinavian study reported that the incidence of aplastic anemia among the Swedish population is 2.3 cases per million individuals per year, with a median age at diagnosis of 60 years and a slight female predominance (52% versus 48%, respectively).2 This data is congruent with prior observations made in Barcelona, where the incidence was 2.34 cases per million individuals per year, albeit with a slightly higher incidence in males compared to females (2.54 versus 2.16, respectively).4 The incidence of aplastic anemia varies globally, with a disproportionate increase in incidence seen among Asian populations, with rates as high as 8.8 per million individuals per year.3-5 This variation in incidence in Asia versus other countries has not been well explained. There appears to be a bimodal distribution, with incidence peaks seen in young adults and in older adults.2,3,6
Pathophysiology
Acquired Aplastic Anemia
The leading hypothesis as to the cause of most cases of acquired aplastic anemia is that a dysregulated immune system destroys HPSCs. Inciting etiologies implicated in the development of acquired aplastic anemia include pregnancy, infection, medications, and exposure to certain chemicals, such as benzene.1,7 The historical understanding of acquired aplastic anemia implicates cytotoxic T-lymphocyte–mediated destruction of CD34+ hematopoietic stem cells.1,8,9 This hypothesis served as the basis for treatment of acquired aplastic anemia with immunosuppressive therapy, predominantly anti-thymocyte globulin (ATG) combined with cyclosporine A.1,8 More recent work has focused on cytokine interactions, particularly the suppressive role of interferon (IFN)-γ on hematopoietic stem cells independent of T-lymphocyte–mediated destruction, which has been demonstrated in a murine model.8 The interaction of IFN-γ with the hematopoietic stem cell pool is dynamic. IFN-γ levels are elevated during an acute inflammatory response, such as a viral infection, providing further basis for the immune-mediated nature of the acquired disease.10 Specifically, in vitro studies suggest the effects of IFN-γ on HPSC may be secondary to interruption of thrombopoietin and its respective signaling pathways, which play a key role in hematopoietic stem cell renewal.11 Eltrombopag, a thrombopoietin receptor antagonist, has shown promise in the treatment of refractory aplastic anemia, with studies indicating that its effectiveness is independent of IFN-γ levels.11,12
Inherited Aplastic Anemia
The inherited marrow failure syndromes (IMFSs) are a group of disorders characterized by cellular maintenance and repair defects, leading to cytopenias, increased cancer risk, structural defects, and risk of end organ damage, such as liver cirrhosis and pulmonary fibrosis.13-15 The most common diseases include Fanconi anemia, dyskeratosis congenita/telomere biology disorders, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, but with the advent of whole exome sequencing, new syndromes continue to be discovered. While classically these disorders present in children, adult presentations are now commonplace. Broadly, the pathophysiology of inherited aplastic anemia relates to the defective HPSCs and an accelerated decline of the hematopoietic stem cell compartment.
The most common IMFSs, Fanconi anemia and telomere biology disorders, are associated with numerous mutations in DNA damage repair pathways and telomere maintenance pathways. TERT, DKC, and TERC mutations are most commonly associated with dyskeratosis congenita, but may also be found infrequently in patients with aplastic anemia presenting at an older age in the absence of the classic phenotypical features.1,16,17 The recognition of an underlying genetic disorder or telomere biology disorder leading to constitutional aplastic anemia is significant, as these conditions are associated not only with marrow failure, but also with endocrinopathies, organ fibrosis, and and hematopoietic and solid organ malignancies.13-15 In particular, TERT and TERC gene mutations have been associated with dyskeratosis congenita as well as pulmonary fibrosis and cirrhosis.18,19 The implications of early diagnosis of an IMFS lie in the approach to treatment and prognosis.
Clonal Disorders and Secondary Malignancies
Myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML) are 2 clonal disorders that may arise from a background of aplastic anemia.9,20,21 Hypoplastic MDS can be difficult to differentiate from aplastic anemia at diagnosis based on morphology alone, although recent work has demonstrated that molecular testing for somatic mutations in ASXL1, DNMT3A, and BCOR can aid in differentiating a subset of aplastic anemia patients who are more likely to progress to MDS.21 Clonal populations of cells harboring 6p uniparental disomy are seen in more than 10% of patients with aplastic anemia on cytogenetic analysis, which can help differentiate the diseases.9 Yoshizato and colleagues found lower rates of ASXL1 and DNMT3A mutations in patients with aplastic anemia as compared with patients with MDS or AML. In this study, patients with aplastic anemia had higher rates of mutations in PIGA (reflecting the increased paroxysmal nocturnal hemoglobinuria [PNH] clonality seen in aplastic anemia) and BCOR.9 Mutations were also found in genes commonly mutated in MDS and AML, including TET2, RUNX1, TP53, and JAK2, albeit at lower frequencies.9 These mutations as a whole have not predicted response to therapy or prognosis. However, when performing survival analysis in patients with specific mutations, those commonly encountered in MDS/AML (ASXL1, DNMT3A, TP53, RUNX1, CSMD1) are associated with faster progression to overt MDS/AML and decreased overall survival (OS),20,21 suggesting these mutations may represent early clonality that can lead to clonal evolution and the development of secondary malignancies. Conversely, mutations in BCOR and BCORL appear to identify patients who may have a favorable outcome in response to immunosuppressive therapy and, similar to patients with PIGA mutations, improved OS.9
Paroxysmal Nocturnal Hemoglobinuria
In addition to having an increased risk of myelodysplasia and malignancy due to the development of a dominant pre-malignant clone, patients with aplastic anemia often harbor progenitor cell clones associated with PNH.1,17 PNH clones have been identified in more than 50% of patients with aplastic anemia.22,23 PNH represents a clonal disorder of hematopoiesis in which cells harbor X-linked somatic mutations in the PIGA gene; this gene encodes a protein responsible for the synthesis of glycosylphosphatidylinositol anchors on the cell surface.22,24 The lack of these cell surface proteins, specifically CD55 (also known as decay accelerating factor) and CD59 (also known as membrane inhibitor of reactive lysis), predisposes red cells to increased complement-mediated lysis.25 The exact mechanism for the development of these clones in patients with aplastic anemia is not fully understood. Current theories hypothesize that the clones are protected from the immune-mediated destruction of normal hematopoietic stem cells due to the absence of the cell surface proteins.1,20 The role of these clones over time in patients with aplastic anemia is less clear, though recent work demonstrated that despite differences in clonality over the disease course, aplastic anemia patients with small PNH clones are less likely to develop overt hemolysis and larger PNH clones compared to patients harboring larger (≥ 50%) PNH clones at diagnosis.23,26,27 Additionally, PNH clones in patients with aplastic anemia infrequently become clinically significant.27 It should be noted that these conditions exist along a continuum; that is, patients with aplastic anemia may develop PNH clones, while conversely patients with PNH may develop aplastic anemia.20 Patients with PNH clones should be followed via peripheral blood flow cytometry and complete blood count to track clonal stability and identify clinically significant PNH among aplastic anemia patients.28
Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying; pulmonary, renal, and liver disease; and blood disorders.
Patients with an IMFS (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, classic phenotypical findings may be lacking in up to 30% to 40% of patients with an IMFS.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered, including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7
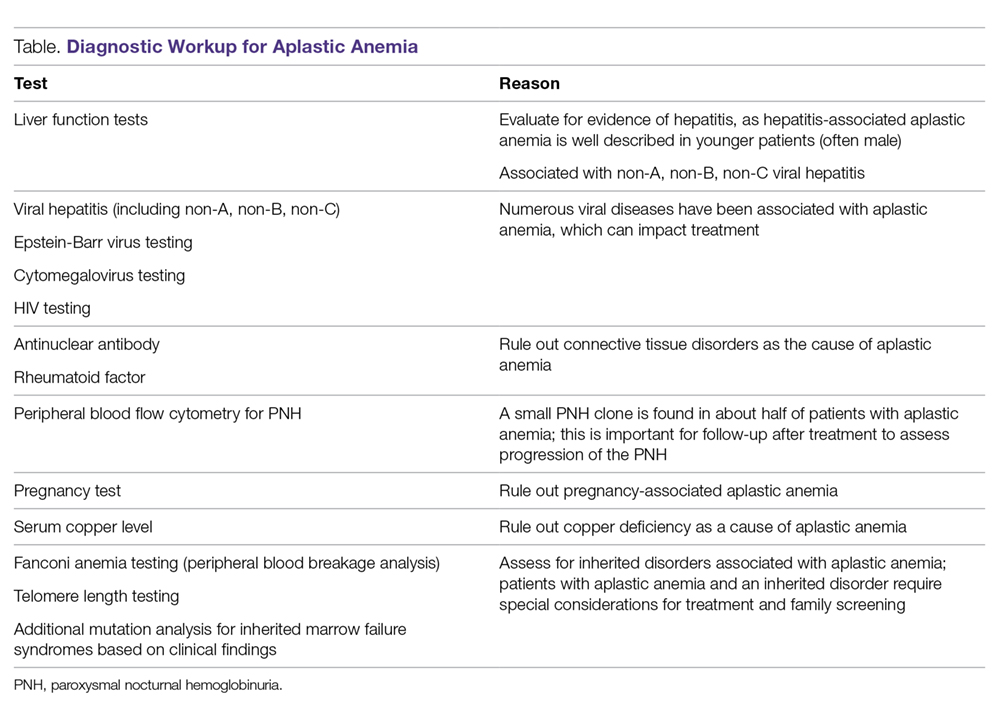
Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 Typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35
In addition to peripheral blood findings, bone marrow biopsy is essential for the diagnosis, and should demonstrate a markedly hypocellular marrow (cellularity < 25%), occasionally with an increase in T lymphocytes.7,39 Because marrow cellularity varies with age and can be challenging to assess, additional biopsies may be needed to confirm the diagnosis.29 A 1- to 2-cm core biopsy is necessary to confirm hypocellularity, as small areas of residual hematopoiesis may be present and obscure the diagnosis.35
Excluding Hypocellular MDS and IMFS
Excluding hypocellular MDS is challenging, especially in the older adult presenting with aplastic anemia, as patients with aplastic anemia may have some degree of erythroid dysplasia on bone marrow morphology.36 The presence of a PNH clone on flow cytometry can aid in diagnosing aplastic anemia and excluding MDS,34 although PNH clones can be present in refractory anemia MDS. Patients with aplastic anemia have a lower ratio of CD34+ cells compared to those with hypoplastic MDS, with 1 study demonstrating a mean CD34+ percentage of < 0.5% in aplastic anemia versus 3.7% in hypoplastic MDS.40 Cytogenetic and molecular testing can also aid in making this distinction by identifying mutations commonly implicated in MDS.7 The presence of monosomy 7 (-7) in aplastic anemia patients is associated with a poor overall prognosis.34,41
Peripheral blood screening using chromosome breakage analysis (done using either mitomycin C or diepoxybutane as in vitro DNA-crosslinking agents)42 and telomere length testing (of peripheral blood leukocytes) is necessary to exclude the main IMFSs, Fanconi anemia and telomere biology disorders, respectively. Ruling out these conditions is imperative, as the approach to treatment varies significantly between IMFS and aplastic anemia. Patients with shortened telomeres should undergo genetic screening for mutations in the telomere maintenance genes to evaluate the underlying defect leading to shortened telomeres. Patients with increased peripheral blood breakage should have genetic testing to detect mutations associated with Fanconi anemia.
Classification
Once the diagnosis of aplastic anemia has been made, the patient should be classified according to the severity of their disease. Disease severity is determined based on peripheral blood ANC: non-severe aplastic anemia (NSAA), ANC > 500 polymorphonuclear neutrophils (PMNs)/µL; severe aplastic anemia (SAA), 200–500 PMNs/µL; and very severe aplastic anemia (VSAA), 0–200 PMNs/µL.4,34 Disease classification is important, as VSAA is associated with a decreased OS compared to SAA.2 Disease classification may affect treatment decisions, as patients with NSAA may be observed for a short period of time, while, conversely, patients with SAA have a worse prognosis with delays in therapy.43-45
Treatment of Inherited Aplastic Anemia
First-line treatment options for patients with IMFS are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect. Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with multiple studies in IMFSs and SAA showing superior outcomes with a bone marrow product compared to peripheral blood stem cells.46-48 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.49,50 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival46,49,51 compared to cyclophosphamide conditioning, which was historically used in matched related donors.50,52 Adding fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.51,53 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data are limited.5
For patients presenting with AML or a high-risk MDS who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggest that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased OS, despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.54,55 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Treatment of Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan are being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.2
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.56,57 All blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.58 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an ANC < 500 cells/µL).59-62 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.63 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006). This difference was largely driven by a decrease in infectious episodes in patients with VSAA treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).63
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.64 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.62,65
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.61,62 This appears to be changing with time. Valdez et al demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.65 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.62 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with SAA or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).60,66 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.60 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues showed that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level of 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).67 Approximately 25% of patients in this trial had an increase in creatinine, with patients taking concomitant cyclosporine affected to a greater degree than those on chelation therapy alone. For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.58
Approach to Therapy
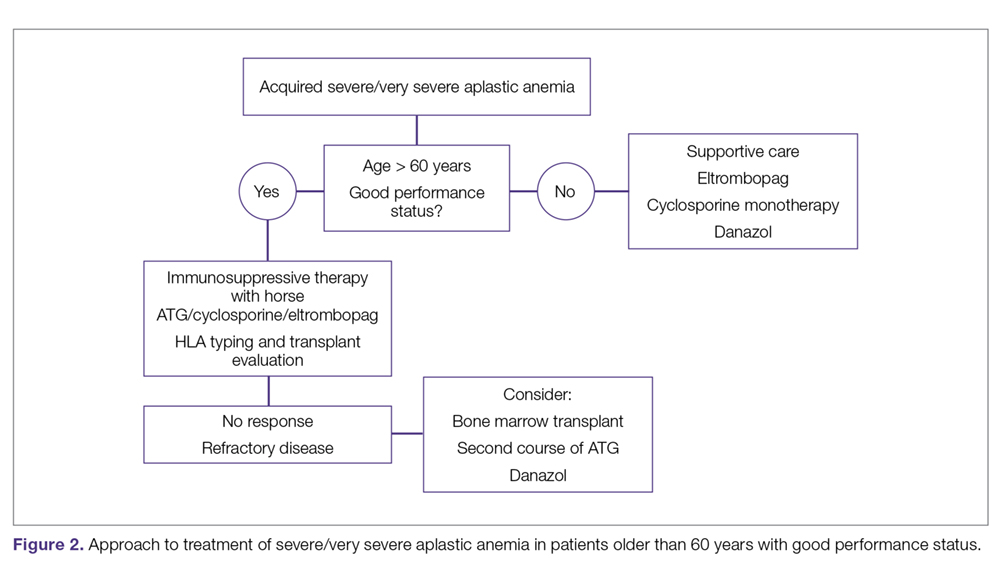
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.
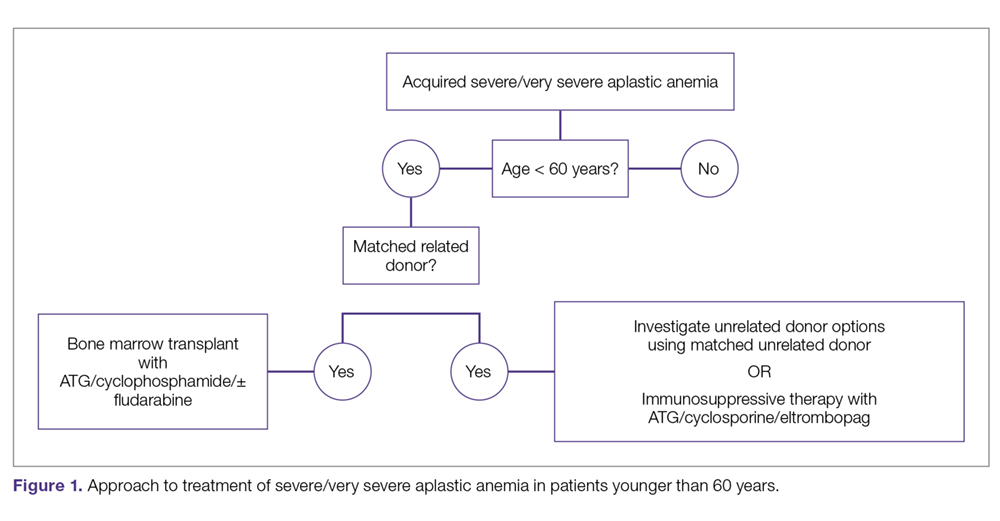
Matched Sibling Donor Transplant. Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).43 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and MUD HSCT (38% and 65%).43 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.68
Current conditioning regimens typically use a combination of cyclophosphamide and ATG,69,70 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.53 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.71
Kim et al evaluated their experience with patients older than 40 years receiving matched related donors, finding comparable outcomes in those ages 41 to 50 years compared to younger patients. Outcomes declined in those over the age of 50 years.72 Long-term data for matched related donor transplant for aplastic anemia show excellent long-term outcomes, with minimal chronic GVHD and good performance status.73 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and MUD transplants for pediatric patients74,75 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.76,77
Immunosuppressive Therapy. For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.78 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.59,78,79
Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.80 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).59 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS, 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.81 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.81 The combination of ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.79 In this study, patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.82 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.83 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.84
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.34,84 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.84 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.85 When given at a dose of 150 mg daily in patients ages 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.45 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.12 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.85 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.86 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.48,65 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant. For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation (EBMT) analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-IV GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Factors associated with improved survival in this analysis include transplant under age 20 years (84% versus 72%), transplant within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and cytomegalovirus-negative donor and recipient as compared to other combinations (82% versus 76%).87 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.34,48 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).75 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.48
A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.75 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical PNH, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.88
With continued improvement of less toxic and more immunomodulating conditioning regimens,utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.89 However, there is still a large population of patients without matched sibling or unrelated donor options. Given the need to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant PNH, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.90
Summary
Aplastic anemia is a rare but potentially life-threatening disorder with pancytopenia and a marked reduction in the HSC compartment. It can be acquired or associated with an IMFS, and the treatment and prognosis vary dramatically between these 2 etiologies. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
Corresponding author: Gabrielle Meyers, MD, 3181 SW Sam Jackson Park Road, Mail Code UHN73C, Portland, OR 97239.
Financial disclosures: None.
1. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519.
2. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
3. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718-1721.
4. Montané E, Ibanez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica. 2008;93:518-523.
5. Ohta A, Nagai M, Nishina M, et al. Incidence of aplastic anemia in Japan: analysis of data from a nationwide registration system. Int J Epidemiol. 2015; 44(suppl_1):i178.
6. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
7. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
8. Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoiesis stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699-3708.
9. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35-47.
10. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479-2486.
11. Cheng H, Cheruku PS, Alvarado L, et al. Interferon-γ perturbs key signaling pathways induced by thrombopoietin, but not eltrombopag, in human hematopoietic stem/progenitor cells. Blood. 2016;128:3870.
12. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
13. Townsley DM, Dumitriu B, Young NS, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922-1931.
14. Feurstein S, Drazer MW, Godley LA. Genetic predisposition to leukemia and other hematologic malignancies. Semin Oncol. 2016;43:598-608.
15. Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775-2783.
16. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16:S119-S125.
17. Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446-4455.
18. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
19. Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J. 2016;48:1721-1731.
20. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337-347.
21. Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood. 2014;124:2698-2704.
22. Mukhina GL, Buckley JT, Barber JP, et al. Multilineage glycosylphosphatidylinositol anchor‐deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476-482.
23. Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45.
24. Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-5340.
25. Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190-198.
26. Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308-1314.
27. Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-1080.
28. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709.
29. Guinan EC. Diagnosis and management of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:76-81.
30. Giampietro PF, Verlander PC, Davis JG, Auerbach AD. Diagnosis of Fanconi anemia in patients without congenital malformations: an international Fanconi Anemia Registry Study. Am J Med Genetics. 1997;68:58-61.
31. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4-10.
32. Giampietro PF, Davis JG, Adler-Brecher B, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116-1120.
33. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
34. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
35. DeZern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol. 2011;4:221-230.
36. Tichelli A, Gratwohl A, Nissen C, et al. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood. 1992;80:337-345.
37. Camitta BM, Storb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645-652.
38. Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177-182.
39. Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136-2141.
40. Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458-462.
41. Maciejewski JP, Risitano AM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129-3135.
42. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
43. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-18.
44. Passweg JR, Socié G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858-864.
45. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95:2119-2125.
46. Peffault de Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122:4279-4286.
47. Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
48. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
49. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Bone Marrow Transplant. 2015;50:1168-1172.
50. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
51. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
52. Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
53. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
54. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-e200.
55. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for Fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
56. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
57. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
58. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
59. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430-438.
60. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
61. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol. 2009;46:269-276.
62. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
63. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
64. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
65. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
66. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
67. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2454.
68. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
69. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
70. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. Hematology Am Soc Hematol Educ Program. 2013;2013:82-86.
71. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
72. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
73. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
74. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
75. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br J Haematol. 2015;171:585-594.
76. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
77. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
78. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
79. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al; German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
80. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
81. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
82. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
83. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
84. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
85. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
86. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018;124:4192-4201.
87. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
88. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. Am J Hematol. 2019; 94:80-86.
89. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
90. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
From the Oregon Health and Science University, Portland, OR.
Abstract
- Objective: To describe the current approach to diagnosis and treatment of aplastic anemia.
- Methods: Review of the literature.
- Results: Aplastic anemia can be acquired or associated with an inherited marrow failure syndrome (IMFS), and the treatment and prognosis vary dramatically between these 2 etiologies. Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to life-threatening neutropenic infections or bleeding. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia.
- Conclusion: Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marro
w transplant program to evaluate for early transplantation is the new standard of care for aplastic anemia.
Keywords: inherited marrow failure syndrome; Fanconi anemia; immunosuppression; transplant; stem cell.
Aplastic anemia is a clinical and pathological entity of bone marrow failure that causes progressive loss of hematopoietic progenitor stem cells (HPSC), resulting in pancytopenia.1 Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to having life-threatening neutropenic infections or bleeding. Aplastic anemia results from either inherited or acquired causes, and the pathophysiology and treatment approach vary significantly between these 2 causes. Therefore, recognition of inherited marrow failure diseases, such as Fanconi anemia and telomere biology disorders, is critical to establishing the management plan.
Epidemiology
Aplastic anemia is a rare disorder, with an incidence of approximately 1.5 to 7 cases per million individuals per year.2,3 A recent Scandinavian study reported that the incidence of aplastic anemia among the Swedish population is 2.3 cases per million individuals per year, with a median age at diagnosis of 60 years and a slight female predominance (52% versus 48%, respectively).2 This data is congruent with prior observations made in Barcelona, where the incidence was 2.34 cases per million individuals per year, albeit with a slightly higher incidence in males compared to females (2.54 versus 2.16, respectively).4 The incidence of aplastic anemia varies globally, with a disproportionate increase in incidence seen among Asian populations, with rates as high as 8.8 per million individuals per year.3-5 This variation in incidence in Asia versus other countries has not been well explained. There appears to be a bimodal distribution, with incidence peaks seen in young adults and in older adults.2,3,6
Pathophysiology
Acquired Aplastic Anemia
The leading hypothesis as to the cause of most cases of acquired aplastic anemia is that a dysregulated immune system destroys HPSCs. Inciting etiologies implicated in the development of acquired aplastic anemia include pregnancy, infection, medications, and exposure to certain chemicals, such as benzene.1,7 The historical understanding of acquired aplastic anemia implicates cytotoxic T-lymphocyte–mediated destruction of CD34+ hematopoietic stem cells.1,8,9 This hypothesis served as the basis for treatment of acquired aplastic anemia with immunosuppressive therapy, predominantly anti-thymocyte globulin (ATG) combined with cyclosporine A.1,8 More recent work has focused on cytokine interactions, particularly the suppressive role of interferon (IFN)-γ on hematopoietic stem cells independent of T-lymphocyte–mediated destruction, which has been demonstrated in a murine model.8 The interaction of IFN-γ with the hematopoietic stem cell pool is dynamic. IFN-γ levels are elevated during an acute inflammatory response, such as a viral infection, providing further basis for the immune-mediated nature of the acquired disease.10 Specifically, in vitro studies suggest the effects of IFN-γ on HPSC may be secondary to interruption of thrombopoietin and its respective signaling pathways, which play a key role in hematopoietic stem cell renewal.11 Eltrombopag, a thrombopoietin receptor antagonist, has shown promise in the treatment of refractory aplastic anemia, with studies indicating that its effectiveness is independent of IFN-γ levels.11,12
Inherited Aplastic Anemia
The inherited marrow failure syndromes (IMFSs) are a group of disorders characterized by cellular maintenance and repair defects, leading to cytopenias, increased cancer risk, structural defects, and risk of end organ damage, such as liver cirrhosis and pulmonary fibrosis.13-15 The most common diseases include Fanconi anemia, dyskeratosis congenita/telomere biology disorders, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, but with the advent of whole exome sequencing, new syndromes continue to be discovered. While classically these disorders present in children, adult presentations are now commonplace. Broadly, the pathophysiology of inherited aplastic anemia relates to the defective HPSCs and an accelerated decline of the hematopoietic stem cell compartment.
The most common IMFSs, Fanconi anemia and telomere biology disorders, are associated with numerous mutations in DNA damage repair pathways and telomere maintenance pathways. TERT, DKC, and TERC mutations are most commonly associated with dyskeratosis congenita, but may also be found infrequently in patients with aplastic anemia presenting at an older age in the absence of the classic phenotypical features.1,16,17 The recognition of an underlying genetic disorder or telomere biology disorder leading to constitutional aplastic anemia is significant, as these conditions are associated not only with marrow failure, but also with endocrinopathies, organ fibrosis, and and hematopoietic and solid organ malignancies.13-15 In particular, TERT and TERC gene mutations have been associated with dyskeratosis congenita as well as pulmonary fibrosis and cirrhosis.18,19 The implications of early diagnosis of an IMFS lie in the approach to treatment and prognosis.
Clonal Disorders and Secondary Malignancies
Myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML) are 2 clonal disorders that may arise from a background of aplastic anemia.9,20,21 Hypoplastic MDS can be difficult to differentiate from aplastic anemia at diagnosis based on morphology alone, although recent work has demonstrated that molecular testing for somatic mutations in ASXL1, DNMT3A, and BCOR can aid in differentiating a subset of aplastic anemia patients who are more likely to progress to MDS.21 Clonal populations of cells harboring 6p uniparental disomy are seen in more than 10% of patients with aplastic anemia on cytogenetic analysis, which can help differentiate the diseases.9 Yoshizato and colleagues found lower rates of ASXL1 and DNMT3A mutations in patients with aplastic anemia as compared with patients with MDS or AML. In this study, patients with aplastic anemia had higher rates of mutations in PIGA (reflecting the increased paroxysmal nocturnal hemoglobinuria [PNH] clonality seen in aplastic anemia) and BCOR.9 Mutations were also found in genes commonly mutated in MDS and AML, including TET2, RUNX1, TP53, and JAK2, albeit at lower frequencies.9 These mutations as a whole have not predicted response to therapy or prognosis. However, when performing survival analysis in patients with specific mutations, those commonly encountered in MDS/AML (ASXL1, DNMT3A, TP53, RUNX1, CSMD1) are associated with faster progression to overt MDS/AML and decreased overall survival (OS),20,21 suggesting these mutations may represent early clonality that can lead to clonal evolution and the development of secondary malignancies. Conversely, mutations in BCOR and BCORL appear to identify patients who may have a favorable outcome in response to immunosuppressive therapy and, similar to patients with PIGA mutations, improved OS.9
Paroxysmal Nocturnal Hemoglobinuria
In addition to having an increased risk of myelodysplasia and malignancy due to the development of a dominant pre-malignant clone, patients with aplastic anemia often harbor progenitor cell clones associated with PNH.1,17 PNH clones have been identified in more than 50% of patients with aplastic anemia.22,23 PNH represents a clonal disorder of hematopoiesis in which cells harbor X-linked somatic mutations in the PIGA gene; this gene encodes a protein responsible for the synthesis of glycosylphosphatidylinositol anchors on the cell surface.22,24 The lack of these cell surface proteins, specifically CD55 (also known as decay accelerating factor) and CD59 (also known as membrane inhibitor of reactive lysis), predisposes red cells to increased complement-mediated lysis.25 The exact mechanism for the development of these clones in patients with aplastic anemia is not fully understood. Current theories hypothesize that the clones are protected from the immune-mediated destruction of normal hematopoietic stem cells due to the absence of the cell surface proteins.1,20 The role of these clones over time in patients with aplastic anemia is less clear, though recent work demonstrated that despite differences in clonality over the disease course, aplastic anemia patients with small PNH clones are less likely to develop overt hemolysis and larger PNH clones compared to patients harboring larger (≥ 50%) PNH clones at diagnosis.23,26,27 Additionally, PNH clones in patients with aplastic anemia infrequently become clinically significant.27 It should be noted that these conditions exist along a continuum; that is, patients with aplastic anemia may develop PNH clones, while conversely patients with PNH may develop aplastic anemia.20 Patients with PNH clones should be followed via peripheral blood flow cytometry and complete blood count to track clonal stability and identify clinically significant PNH among aplastic anemia patients.28
Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying; pulmonary, renal, and liver disease; and blood disorders.
Patients with an IMFS (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, classic phenotypical findings may be lacking in up to 30% to 40% of patients with an IMFS.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered, including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7

Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 Typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35
In addition to peripheral blood findings, bone marrow biopsy is essential for the diagnosis, and should demonstrate a markedly hypocellular marrow (cellularity < 25%), occasionally with an increase in T lymphocytes.7,39 Because marrow cellularity varies with age and can be challenging to assess, additional biopsies may be needed to confirm the diagnosis.29 A 1- to 2-cm core biopsy is necessary to confirm hypocellularity, as small areas of residual hematopoiesis may be present and obscure the diagnosis.35
Excluding Hypocellular MDS and IMFS
Excluding hypocellular MDS is challenging, especially in the older adult presenting with aplastic anemia, as patients with aplastic anemia may have some degree of erythroid dysplasia on bone marrow morphology.36 The presence of a PNH clone on flow cytometry can aid in diagnosing aplastic anemia and excluding MDS,34 although PNH clones can be present in refractory anemia MDS. Patients with aplastic anemia have a lower ratio of CD34+ cells compared to those with hypoplastic MDS, with 1 study demonstrating a mean CD34+ percentage of < 0.5% in aplastic anemia versus 3.7% in hypoplastic MDS.40 Cytogenetic and molecular testing can also aid in making this distinction by identifying mutations commonly implicated in MDS.7 The presence of monosomy 7 (-7) in aplastic anemia patients is associated with a poor overall prognosis.34,41
Peripheral blood screening using chromosome breakage analysis (done using either mitomycin C or diepoxybutane as in vitro DNA-crosslinking agents)42 and telomere length testing (of peripheral blood leukocytes) is necessary to exclude the main IMFSs, Fanconi anemia and telomere biology disorders, respectively. Ruling out these conditions is imperative, as the approach to treatment varies significantly between IMFS and aplastic anemia. Patients with shortened telomeres should undergo genetic screening for mutations in the telomere maintenance genes to evaluate the underlying defect leading to shortened telomeres. Patients with increased peripheral blood breakage should have genetic testing to detect mutations associated with Fanconi anemia.
Classification
Once the diagnosis of aplastic anemia has been made, the patient should be classified according to the severity of their disease. Disease severity is determined based on peripheral blood ANC: non-severe aplastic anemia (NSAA), ANC > 500 polymorphonuclear neutrophils (PMNs)/µL; severe aplastic anemia (SAA), 200–500 PMNs/µL; and very severe aplastic anemia (VSAA), 0–200 PMNs/µL.4,34 Disease classification is important, as VSAA is associated with a decreased OS compared to SAA.2 Disease classification may affect treatment decisions, as patients with NSAA may be observed for a short period of time, while, conversely, patients with SAA have a worse prognosis with delays in therapy.43-45
Treatment of Inherited Aplastic Anemia
First-line treatment options for patients with IMFS are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect. Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with multiple studies in IMFSs and SAA showing superior outcomes with a bone marrow product compared to peripheral blood stem cells.46-48 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.49,50 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival46,49,51 compared to cyclophosphamide conditioning, which was historically used in matched related donors.50,52 Adding fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.51,53 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data are limited.5
For patients presenting with AML or a high-risk MDS who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggest that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased OS, despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.54,55 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Treatment of Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan are being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.2
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.56,57 All blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.58 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an ANC < 500 cells/µL).59-62 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.63 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006). This difference was largely driven by a decrease in infectious episodes in patients with VSAA treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).63
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.64 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.62,65
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.61,62 This appears to be changing with time. Valdez et al demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.65 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.62 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with SAA or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).60,66 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.60 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues showed that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level of 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).67 Approximately 25% of patients in this trial had an increase in creatinine, with patients taking concomitant cyclosporine affected to a greater degree than those on chelation therapy alone. For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.58
Approach to Therapy
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.

Matched Sibling Donor Transplant. Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).43 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and MUD HSCT (38% and 65%).43 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.68

Current conditioning regimens typically use a combination of cyclophosphamide and ATG,69,70 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.53 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.71
Kim et al evaluated their experience with patients older than 40 years receiving matched related donors, finding comparable outcomes in those ages 41 to 50 years compared to younger patients. Outcomes declined in those over the age of 50 years.72 Long-term data for matched related donor transplant for aplastic anemia show excellent long-term outcomes, with minimal chronic GVHD and good performance status.73 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and MUD transplants for pediatric patients74,75 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.76,77
Immunosuppressive Therapy. For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.78 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.59,78,79
Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.80 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).59 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS, 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.81 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.81 The combination of ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.79 In this study, patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.82 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.83 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.84
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.34,84 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.84 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.85 When given at a dose of 150 mg daily in patients ages 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.45 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.12 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.85 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.86 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.48,65 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant. For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation (EBMT) analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-IV GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Factors associated with improved survival in this analysis include transplant under age 20 years (84% versus 72%), transplant within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and cytomegalovirus-negative donor and recipient as compared to other combinations (82% versus 76%).87 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.34,48 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).75 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.48
A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.75 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical PNH, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.88
With continued improvement of less toxic and more immunomodulating conditioning regimens,utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.89 However, there is still a large population of patients without matched sibling or unrelated donor options. Given the need to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant PNH, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.90
Summary
Aplastic anemia is a rare but potentially life-threatening disorder with pancytopenia and a marked reduction in the HSC compartment. It can be acquired or associated with an IMFS, and the treatment and prognosis vary dramatically between these 2 etiologies. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
Corresponding author: Gabrielle Meyers, MD, 3181 SW Sam Jackson Park Road, Mail Code UHN73C, Portland, OR 97239.
Financial disclosures: None.
From the Oregon Health and Science University, Portland, OR.
Abstract
- Objective: To describe the current approach to diagnosis and treatment of aplastic anemia.
- Methods: Review of the literature.
- Results: Aplastic anemia can be acquired or associated with an inherited marrow failure syndrome (IMFS), and the treatment and prognosis vary dramatically between these 2 etiologies. Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to life-threatening neutropenic infections or bleeding. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia.
- Conclusion: Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marro
w transplant program to evaluate for early transplantation is the new standard of care for aplastic anemia.
Keywords: inherited marrow failure syndrome; Fanconi anemia; immunosuppression; transplant; stem cell.
Aplastic anemia is a clinical and pathological entity of bone marrow failure that causes progressive loss of hematopoietic progenitor stem cells (HPSC), resulting in pancytopenia.1 Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to having life-threatening neutropenic infections or bleeding. Aplastic anemia results from either inherited or acquired causes, and the pathophysiology and treatment approach vary significantly between these 2 causes. Therefore, recognition of inherited marrow failure diseases, such as Fanconi anemia and telomere biology disorders, is critical to establishing the management plan.
Epidemiology
Aplastic anemia is a rare disorder, with an incidence of approximately 1.5 to 7 cases per million individuals per year.2,3 A recent Scandinavian study reported that the incidence of aplastic anemia among the Swedish population is 2.3 cases per million individuals per year, with a median age at diagnosis of 60 years and a slight female predominance (52% versus 48%, respectively).2 This data is congruent with prior observations made in Barcelona, where the incidence was 2.34 cases per million individuals per year, albeit with a slightly higher incidence in males compared to females (2.54 versus 2.16, respectively).4 The incidence of aplastic anemia varies globally, with a disproportionate increase in incidence seen among Asian populations, with rates as high as 8.8 per million individuals per year.3-5 This variation in incidence in Asia versus other countries has not been well explained. There appears to be a bimodal distribution, with incidence peaks seen in young adults and in older adults.2,3,6
Pathophysiology
Acquired Aplastic Anemia
The leading hypothesis as to the cause of most cases of acquired aplastic anemia is that a dysregulated immune system destroys HPSCs. Inciting etiologies implicated in the development of acquired aplastic anemia include pregnancy, infection, medications, and exposure to certain chemicals, such as benzene.1,7 The historical understanding of acquired aplastic anemia implicates cytotoxic T-lymphocyte–mediated destruction of CD34+ hematopoietic stem cells.1,8,9 This hypothesis served as the basis for treatment of acquired aplastic anemia with immunosuppressive therapy, predominantly anti-thymocyte globulin (ATG) combined with cyclosporine A.1,8 More recent work has focused on cytokine interactions, particularly the suppressive role of interferon (IFN)-γ on hematopoietic stem cells independent of T-lymphocyte–mediated destruction, which has been demonstrated in a murine model.8 The interaction of IFN-γ with the hematopoietic stem cell pool is dynamic. IFN-γ levels are elevated during an acute inflammatory response, such as a viral infection, providing further basis for the immune-mediated nature of the acquired disease.10 Specifically, in vitro studies suggest the effects of IFN-γ on HPSC may be secondary to interruption of thrombopoietin and its respective signaling pathways, which play a key role in hematopoietic stem cell renewal.11 Eltrombopag, a thrombopoietin receptor antagonist, has shown promise in the treatment of refractory aplastic anemia, with studies indicating that its effectiveness is independent of IFN-γ levels.11,12
Inherited Aplastic Anemia
The inherited marrow failure syndromes (IMFSs) are a group of disorders characterized by cellular maintenance and repair defects, leading to cytopenias, increased cancer risk, structural defects, and risk of end organ damage, such as liver cirrhosis and pulmonary fibrosis.13-15 The most common diseases include Fanconi anemia, dyskeratosis congenita/telomere biology disorders, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, but with the advent of whole exome sequencing, new syndromes continue to be discovered. While classically these disorders present in children, adult presentations are now commonplace. Broadly, the pathophysiology of inherited aplastic anemia relates to the defective HPSCs and an accelerated decline of the hematopoietic stem cell compartment.
The most common IMFSs, Fanconi anemia and telomere biology disorders, are associated with numerous mutations in DNA damage repair pathways and telomere maintenance pathways. TERT, DKC, and TERC mutations are most commonly associated with dyskeratosis congenita, but may also be found infrequently in patients with aplastic anemia presenting at an older age in the absence of the classic phenotypical features.1,16,17 The recognition of an underlying genetic disorder or telomere biology disorder leading to constitutional aplastic anemia is significant, as these conditions are associated not only with marrow failure, but also with endocrinopathies, organ fibrosis, and and hematopoietic and solid organ malignancies.13-15 In particular, TERT and TERC gene mutations have been associated with dyskeratosis congenita as well as pulmonary fibrosis and cirrhosis.18,19 The implications of early diagnosis of an IMFS lie in the approach to treatment and prognosis.
Clonal Disorders and Secondary Malignancies
Myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML) are 2 clonal disorders that may arise from a background of aplastic anemia.9,20,21 Hypoplastic MDS can be difficult to differentiate from aplastic anemia at diagnosis based on morphology alone, although recent work has demonstrated that molecular testing for somatic mutations in ASXL1, DNMT3A, and BCOR can aid in differentiating a subset of aplastic anemia patients who are more likely to progress to MDS.21 Clonal populations of cells harboring 6p uniparental disomy are seen in more than 10% of patients with aplastic anemia on cytogenetic analysis, which can help differentiate the diseases.9 Yoshizato and colleagues found lower rates of ASXL1 and DNMT3A mutations in patients with aplastic anemia as compared with patients with MDS or AML. In this study, patients with aplastic anemia had higher rates of mutations in PIGA (reflecting the increased paroxysmal nocturnal hemoglobinuria [PNH] clonality seen in aplastic anemia) and BCOR.9 Mutations were also found in genes commonly mutated in MDS and AML, including TET2, RUNX1, TP53, and JAK2, albeit at lower frequencies.9 These mutations as a whole have not predicted response to therapy or prognosis. However, when performing survival analysis in patients with specific mutations, those commonly encountered in MDS/AML (ASXL1, DNMT3A, TP53, RUNX1, CSMD1) are associated with faster progression to overt MDS/AML and decreased overall survival (OS),20,21 suggesting these mutations may represent early clonality that can lead to clonal evolution and the development of secondary malignancies. Conversely, mutations in BCOR and BCORL appear to identify patients who may have a favorable outcome in response to immunosuppressive therapy and, similar to patients with PIGA mutations, improved OS.9
Paroxysmal Nocturnal Hemoglobinuria
In addition to having an increased risk of myelodysplasia and malignancy due to the development of a dominant pre-malignant clone, patients with aplastic anemia often harbor progenitor cell clones associated with PNH.1,17 PNH clones have been identified in more than 50% of patients with aplastic anemia.22,23 PNH represents a clonal disorder of hematopoiesis in which cells harbor X-linked somatic mutations in the PIGA gene; this gene encodes a protein responsible for the synthesis of glycosylphosphatidylinositol anchors on the cell surface.22,24 The lack of these cell surface proteins, specifically CD55 (also known as decay accelerating factor) and CD59 (also known as membrane inhibitor of reactive lysis), predisposes red cells to increased complement-mediated lysis.25 The exact mechanism for the development of these clones in patients with aplastic anemia is not fully understood. Current theories hypothesize that the clones are protected from the immune-mediated destruction of normal hematopoietic stem cells due to the absence of the cell surface proteins.1,20 The role of these clones over time in patients with aplastic anemia is less clear, though recent work demonstrated that despite differences in clonality over the disease course, aplastic anemia patients with small PNH clones are less likely to develop overt hemolysis and larger PNH clones compared to patients harboring larger (≥ 50%) PNH clones at diagnosis.23,26,27 Additionally, PNH clones in patients with aplastic anemia infrequently become clinically significant.27 It should be noted that these conditions exist along a continuum; that is, patients with aplastic anemia may develop PNH clones, while conversely patients with PNH may develop aplastic anemia.20 Patients with PNH clones should be followed via peripheral blood flow cytometry and complete blood count to track clonal stability and identify clinically significant PNH among aplastic anemia patients.28
Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying; pulmonary, renal, and liver disease; and blood disorders.
Patients with an IMFS (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, classic phenotypical findings may be lacking in up to 30% to 40% of patients with an IMFS.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered, including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7

Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 Typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35
In addition to peripheral blood findings, bone marrow biopsy is essential for the diagnosis, and should demonstrate a markedly hypocellular marrow (cellularity < 25%), occasionally with an increase in T lymphocytes.7,39 Because marrow cellularity varies with age and can be challenging to assess, additional biopsies may be needed to confirm the diagnosis.29 A 1- to 2-cm core biopsy is necessary to confirm hypocellularity, as small areas of residual hematopoiesis may be present and obscure the diagnosis.35
Excluding Hypocellular MDS and IMFS
Excluding hypocellular MDS is challenging, especially in the older adult presenting with aplastic anemia, as patients with aplastic anemia may have some degree of erythroid dysplasia on bone marrow morphology.36 The presence of a PNH clone on flow cytometry can aid in diagnosing aplastic anemia and excluding MDS,34 although PNH clones can be present in refractory anemia MDS. Patients with aplastic anemia have a lower ratio of CD34+ cells compared to those with hypoplastic MDS, with 1 study demonstrating a mean CD34+ percentage of < 0.5% in aplastic anemia versus 3.7% in hypoplastic MDS.40 Cytogenetic and molecular testing can also aid in making this distinction by identifying mutations commonly implicated in MDS.7 The presence of monosomy 7 (-7) in aplastic anemia patients is associated with a poor overall prognosis.34,41
Peripheral blood screening using chromosome breakage analysis (done using either mitomycin C or diepoxybutane as in vitro DNA-crosslinking agents)42 and telomere length testing (of peripheral blood leukocytes) is necessary to exclude the main IMFSs, Fanconi anemia and telomere biology disorders, respectively. Ruling out these conditions is imperative, as the approach to treatment varies significantly between IMFS and aplastic anemia. Patients with shortened telomeres should undergo genetic screening for mutations in the telomere maintenance genes to evaluate the underlying defect leading to shortened telomeres. Patients with increased peripheral blood breakage should have genetic testing to detect mutations associated with Fanconi anemia.
Classification
Once the diagnosis of aplastic anemia has been made, the patient should be classified according to the severity of their disease. Disease severity is determined based on peripheral blood ANC: non-severe aplastic anemia (NSAA), ANC > 500 polymorphonuclear neutrophils (PMNs)/µL; severe aplastic anemia (SAA), 200–500 PMNs/µL; and very severe aplastic anemia (VSAA), 0–200 PMNs/µL.4,34 Disease classification is important, as VSAA is associated with a decreased OS compared to SAA.2 Disease classification may affect treatment decisions, as patients with NSAA may be observed for a short period of time, while, conversely, patients with SAA have a worse prognosis with delays in therapy.43-45
Treatment of Inherited Aplastic Anemia
First-line treatment options for patients with IMFS are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect. Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with multiple studies in IMFSs and SAA showing superior outcomes with a bone marrow product compared to peripheral blood stem cells.46-48 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.49,50 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival46,49,51 compared to cyclophosphamide conditioning, which was historically used in matched related donors.50,52 Adding fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.51,53 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data are limited.5
For patients presenting with AML or a high-risk MDS who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggest that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased OS, despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.54,55 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Treatment of Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan are being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.2
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.56,57 All blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.58 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an ANC < 500 cells/µL).59-62 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.63 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006). This difference was largely driven by a decrease in infectious episodes in patients with VSAA treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).63
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.64 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.62,65
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.61,62 This appears to be changing with time. Valdez et al demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.65 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.62 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with SAA or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).60,66 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.60 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues showed that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level of 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).67 Approximately 25% of patients in this trial had an increase in creatinine, with patients taking concomitant cyclosporine affected to a greater degree than those on chelation therapy alone. For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.58
Approach to Therapy
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.

Matched Sibling Donor Transplant. Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).43 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and MUD HSCT (38% and 65%).43 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.68

Current conditioning regimens typically use a combination of cyclophosphamide and ATG,69,70 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.53 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.71
Kim et al evaluated their experience with patients older than 40 years receiving matched related donors, finding comparable outcomes in those ages 41 to 50 years compared to younger patients. Outcomes declined in those over the age of 50 years.72 Long-term data for matched related donor transplant for aplastic anemia show excellent long-term outcomes, with minimal chronic GVHD and good performance status.73 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and MUD transplants for pediatric patients74,75 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.76,77
Immunosuppressive Therapy. For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.78 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.59,78,79
Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.80 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).59 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS, 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.81 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.81 The combination of ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.79 In this study, patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.82 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.83 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.84
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.34,84 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.84 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.85 When given at a dose of 150 mg daily in patients ages 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.45 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.12 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.85 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.86 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.48,65 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant. For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation (EBMT) analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-IV GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Factors associated with improved survival in this analysis include transplant under age 20 years (84% versus 72%), transplant within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and cytomegalovirus-negative donor and recipient as compared to other combinations (82% versus 76%).87 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.34,48 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).75 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.48
A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.75 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical PNH, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.88
With continued improvement of less toxic and more immunomodulating conditioning regimens,utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.89 However, there is still a large population of patients without matched sibling or unrelated donor options. Given the need to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant PNH, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.90
Summary
Aplastic anemia is a rare but potentially life-threatening disorder with pancytopenia and a marked reduction in the HSC compartment. It can be acquired or associated with an IMFS, and the treatment and prognosis vary dramatically between these 2 etiologies. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
Corresponding author: Gabrielle Meyers, MD, 3181 SW Sam Jackson Park Road, Mail Code UHN73C, Portland, OR 97239.
Financial disclosures: None.
1. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519.
2. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
3. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718-1721.
4. Montané E, Ibanez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica. 2008;93:518-523.
5. Ohta A, Nagai M, Nishina M, et al. Incidence of aplastic anemia in Japan: analysis of data from a nationwide registration system. Int J Epidemiol. 2015; 44(suppl_1):i178.
6. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
7. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
8. Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoiesis stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699-3708.
9. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35-47.
10. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479-2486.
11. Cheng H, Cheruku PS, Alvarado L, et al. Interferon-γ perturbs key signaling pathways induced by thrombopoietin, but not eltrombopag, in human hematopoietic stem/progenitor cells. Blood. 2016;128:3870.
12. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
13. Townsley DM, Dumitriu B, Young NS, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922-1931.
14. Feurstein S, Drazer MW, Godley LA. Genetic predisposition to leukemia and other hematologic malignancies. Semin Oncol. 2016;43:598-608.
15. Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775-2783.
16. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16:S119-S125.
17. Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446-4455.
18. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
19. Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J. 2016;48:1721-1731.
20. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337-347.
21. Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood. 2014;124:2698-2704.
22. Mukhina GL, Buckley JT, Barber JP, et al. Multilineage glycosylphosphatidylinositol anchor‐deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476-482.
23. Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45.
24. Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-5340.
25. Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190-198.
26. Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308-1314.
27. Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-1080.
28. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709.
29. Guinan EC. Diagnosis and management of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:76-81.
30. Giampietro PF, Verlander PC, Davis JG, Auerbach AD. Diagnosis of Fanconi anemia in patients without congenital malformations: an international Fanconi Anemia Registry Study. Am J Med Genetics. 1997;68:58-61.
31. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4-10.
32. Giampietro PF, Davis JG, Adler-Brecher B, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116-1120.
33. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
34. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
35. DeZern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol. 2011;4:221-230.
36. Tichelli A, Gratwohl A, Nissen C, et al. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood. 1992;80:337-345.
37. Camitta BM, Storb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645-652.
38. Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177-182.
39. Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136-2141.
40. Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458-462.
41. Maciejewski JP, Risitano AM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129-3135.
42. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
43. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-18.
44. Passweg JR, Socié G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858-864.
45. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95:2119-2125.
46. Peffault de Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122:4279-4286.
47. Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
48. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
49. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Bone Marrow Transplant. 2015;50:1168-1172.
50. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
51. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
52. Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
53. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
54. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-e200.
55. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for Fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
56. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
57. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
58. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
59. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430-438.
60. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
61. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol. 2009;46:269-276.
62. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
63. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
64. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
65. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
66. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
67. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2454.
68. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
69. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
70. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. Hematology Am Soc Hematol Educ Program. 2013;2013:82-86.
71. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
72. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
73. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
74. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
75. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br J Haematol. 2015;171:585-594.
76. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
77. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
78. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
79. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al; German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
80. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
81. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
82. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
83. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
84. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
85. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
86. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018;124:4192-4201.
87. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
88. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. Am J Hematol. 2019; 94:80-86.
89. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
90. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
1. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519.
2. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
3. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718-1721.
4. Montané E, Ibanez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica. 2008;93:518-523.
5. Ohta A, Nagai M, Nishina M, et al. Incidence of aplastic anemia in Japan: analysis of data from a nationwide registration system. Int J Epidemiol. 2015; 44(suppl_1):i178.
6. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
7. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
8. Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoiesis stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699-3708.
9. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35-47.
10. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479-2486.
11. Cheng H, Cheruku PS, Alvarado L, et al. Interferon-γ perturbs key signaling pathways induced by thrombopoietin, but not eltrombopag, in human hematopoietic stem/progenitor cells. Blood. 2016;128:3870.
12. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
13. Townsley DM, Dumitriu B, Young NS, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922-1931.
14. Feurstein S, Drazer MW, Godley LA. Genetic predisposition to leukemia and other hematologic malignancies. Semin Oncol. 2016;43:598-608.
15. Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775-2783.
16. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16:S119-S125.
17. Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446-4455.
18. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
19. Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J. 2016;48:1721-1731.
20. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337-347.
21. Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood. 2014;124:2698-2704.
22. Mukhina GL, Buckley JT, Barber JP, et al. Multilineage glycosylphosphatidylinositol anchor‐deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476-482.
23. Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45.
24. Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-5340.
25. Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190-198.
26. Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308-1314.
27. Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-1080.
28. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709.
29. Guinan EC. Diagnosis and management of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:76-81.
30. Giampietro PF, Verlander PC, Davis JG, Auerbach AD. Diagnosis of Fanconi anemia in patients without congenital malformations: an international Fanconi Anemia Registry Study. Am J Med Genetics. 1997;68:58-61.
31. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4-10.
32. Giampietro PF, Davis JG, Adler-Brecher B, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116-1120.
33. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
34. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
35. DeZern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol. 2011;4:221-230.
36. Tichelli A, Gratwohl A, Nissen C, et al. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood. 1992;80:337-345.
37. Camitta BM, Storb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645-652.
38. Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177-182.
39. Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136-2141.
40. Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458-462.
41. Maciejewski JP, Risitano AM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129-3135.
42. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
43. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-18.
44. Passweg JR, Socié G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858-864.
45. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95:2119-2125.
46. Peffault de Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122:4279-4286.
47. Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
48. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
49. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Bone Marrow Transplant. 2015;50:1168-1172.
50. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
51. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
52. Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
53. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
54. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-e200.
55. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for Fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
56. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
57. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
58. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
59. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430-438.
60. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
61. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol. 2009;46:269-276.
62. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
63. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
64. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
65. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
66. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
67. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2454.
68. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
69. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
70. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. Hematology Am Soc Hematol Educ Program. 2013;2013:82-86.
71. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
72. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
73. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
74. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
75. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br J Haematol. 2015;171:585-594.
76. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
77. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
78. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
79. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al; German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
80. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
81. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
82. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
83. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
84. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
85. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
86. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018;124:4192-4201.
87. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
88. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. Am J Hematol. 2019; 94:80-86.
89. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
90. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.