User login
In March 2016, 13 practices affiliated with the US Oncology Network (USON) were invited to participate in the Oncology Care Model (OCM) proposed by the Center for Medicare and Medicaid Services (CMS) and Center for Medicare and Medicaid Innovation (CMMI). The OCM, a novel value-based care model, was designed to provide higher-quality and better-coordinated oncology care at a lower cost to CMS.1 Of the 13 practices, 12 agreed to participate with a start date for the program of July 1, 2016. At least 40% of the practices’ patients were insured by Medicare, and any eligible patients with active cancer were offered an opportunity to enter the program. USON practices treat more than 25,000 patients with a qualifying episode per year and the overall OCM program sees more than 150,000 beneficiaries per year,2 so we anticipated that the OCM would have a substantial impact on each of the 12 practices on USON.
Faced with the scenario of having only 3 months between notification of approval and launch of the OCM, it was imperative that all the practices be proactive in planning and preparing to launch the OCM. With this goal in mind, representatives from all OCM candidate practices convened to anticipate the needs of the OCM and chart out a program to meet those needs. In this article, we discuss the requirements and scope of the OCM, the development of an internal pilot project, the anticipated gains from the pilot, and the results and findings from the pilot, both expected and unexpected.
The road to the Oncology Care Model
The government and oncology practices have been on separate trajectories to the OCM. In the last 15 years, the major intersections of these trajectories had to do with price and not patient outcomes. In 2003, the Medicare Prescription Drug Improvement and Modernization Act (MMA) focused on drug price reductions from an average wholesale price–based schedule to an average sales price–based schedule.3 There was the sequester in 2013,4 and more recently a proposal to restructure the payment for Part B drugs. In the background, recurrent negotiations to fix the calculation for the sustainable growth rate allowed for periodic draconian cuts to the prices of services. The cumulative effect of these price reductions has been to put economic pressure on community oncologists such that many have moved to a hospital environment.5
This contentious relationship with community oncology began to change with the passage of the Affordable Care Act (ACA) in 2010.6 Section 3022 of the ACA established the Medicare Shared Savings Program (MSSP) with the charge to create a new type of health care entity that was responsible for achieving the triple aim of improving population health, improving individual patient care, and bending the cost curve.7 Additional programs, such as the Pioneer Accountable Care Organization (ACO) program and the Comprehensive Primary Care Initiative were established to test alternative payment models.8-10
The ACA also funded the CMMI with a mandate to “test innovative payment and service delivery models” to achieve the triple aim; US$10 billion were appropriated for the years 2011-2019 for this purpose. The CMMI funded a pilot project for cancer care, the COME HOME [Community Oncology MEdical HOME] initiative, to test whether some aspects of care could be transformed or augmented to reduce overall costs or at least reduce the rate of increase. Findings from COME HOME have helped inform the OCM program.11
Over the same period, practices belonging to the USON were paving a path toward value. An electronic health record (EHR) for the entire network was adopted in 2005. A pathways program in which chemotherapy regimens were assessed on cost as well as benefits and toxicity, was started in 2006. Higher-cost regimens with no additional benefits comparable with other evidence-based regimens were deselected for initial treatment choices at the time of initial decision support. This process was streamlined using web-based technology that improved pathways compliance and tracking of off-pathways exceptions.12 Retrospective studies indicated that pathways had the potential to bend the cost curve by reducing drug spending.13,14 USON and its practices also tested a nurse call system (Innovent Oncology) funded by a monthly management fee. This program guided patients through chemotherapy with regular telephonic symptom assessment and discussion of patient-centered values and advance care planning. Results of these programs indicated relative reductions in both drug and hospital expenses.15
Additional experience has come from participation in the United Healthcare Episodes of Care (EOC) initiative, which eliminated the chemotherapy drug incentives, compensating physicians on a per-episode basis instead. This study showed a significant reduction in the total cost of cancer therapy after modifying the fee-for-service system and incorporating feedback data and financial incentives to reward improved outcomes and cost efficiency.16
The Oncology Care Model represents a convergence of purchaser demand and provider readiness. The purchaser holds providers accountable for cost and quality. The data on outcomes and costs will provide an extensive database that can be analyzed by the participating practices to address variations and reduce unnecessary care and preventable costs. Best practices are rewarded.17
The OCM and practice readiness
As a part of the CMS proposal process, practices were required to submit implementation plans by June 30, 2015. The purpose of the implementation plan was to define how a practice could transform over 6 broad domains: 24/7 coverage; EHR certification; navigation and care coordination; continuous quality improvement; incorporation of the Institute of Medicine’s (IOM’s) Care Management Plan; and adherence to nationally recognized guidelines. The periods of patient eligibility for the program were 6-month treatment episodes triggered by a cancer diagnosis, a provider encounter claim, and a Part B or D drug claim specifically identified as a cancer treatment. The episodes could be repeated if the 3 criteria continued to be met. All charges continued to be billed as fee-for-service as before, but in addition, participating practices could bill a monthly enhanced oncology services (MEOS) payment for the duration of an episode. Reducing the total cost of care while meeting performance metrics thresholds would also qualify a practice for performance-based payments.
Of the primary components, EHR certification and adherence to guidelines had been addressed previously, but the other domains represented significant challenges. Although 24/7 physician coverage with access to an EHR is standard for all practices, most practice sites do not have an insight into the frequency of hospital admissions, the ability to efficiently add sick patients to the daily schedule, or a routine call system to assess chemotherapy toxicity.
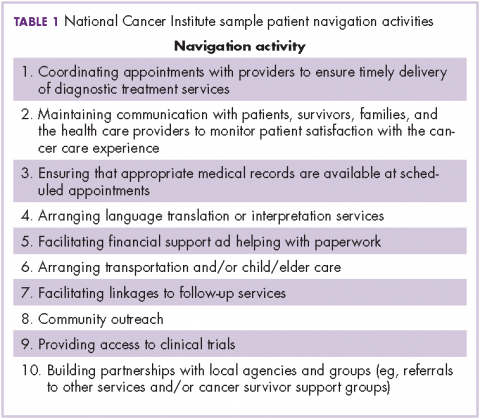
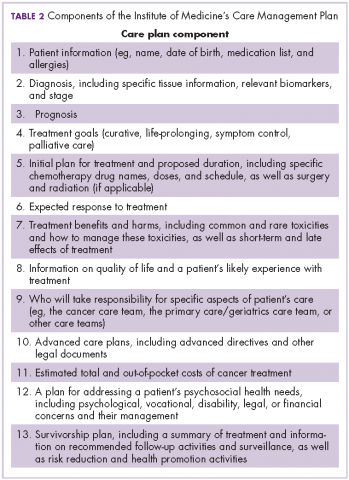
The OCM proposes 10 potential navigation/care coordination functions (Table 1) and does not consider those functions to be the role of one person, but rather a team responsibility. Most of our practices perform at least some of these functions, but they are not formally designated, coordinated, or recorded. A similar condition exists for the IOM care plan, which includes recommendations for treatment and adverse event management (Table 2). The prognosis, toxicity, quality-of-life, and goals-of-care requirements are often found in the physician notes, but not systematically documented or searchable.
Similar challenges have been observed for continuous quality improvement programs. Although the data are available, they are often not easy to search and, therefore, are difficult to retrieve and report. The OCM, as with any transformational program, must always weigh the benefit of information with the burden of consumption of physician and staff time to collect and input these data.18
Prepilot project work
In October 2015, lead physicians and managers from the 12 participating practices were brought together with analytic, technical, process management, and business experts from USON and McKesson Specialty Health. The objective of the meeting was to define the areas of greatest need for day 1 of the OCM and to be prepared. The challenges were to identify the changes needed to meet the requirements of the OCM while improving the patient experience, sustaining the viability of the community oncology practices, creating teams to deliver more effective care, and using data to bend the cost curve. Accordingly, 4 work streams were created: Care and Support; Content; Technology; and Communications, Revenue Cycle and Incentives.
Care and Support
The key tasks of the Care and Support team were to define the workflows for navigation and the IOM care plan. As a patient’s journey through the clinic was mapped out, it became clear that although multiple personnel could participate in the navigation and care plans, there was no systematic way to organize and record the components of successful navigation. The goals for the pilot were to test various options for navigation and to identify best practices that could be translated into standard operating procedures.
Content
The Content team was charged with identifying available programs that would fit into the OCM requirements. These included advance care planning, survivorship, chemotherapy teaching, risk assessment, pathways, and symptom assessment. A longer-term goal was the development of care paths, a more comprehensive map of the patient’s journey that would include consultations, coordinated care, imaging, labs, and other services.
Technology
The task for the Technology work stream was to identify processes of care that required documentation and to evaluate current and future technology solutions to improve efficiencies. The electronic medical record satisfied for the input of data with relevant clinical details, demographics, disease types, and staging. A web-based pathways tool supported clinical decision-making, as well as compliance to pathways. The Medicare quality metric programs set the stage for development of capture and reporting tools for data from many sources. The pilot would indicate the adequacy of these tools and the need for expansion or development of new functions or programs. Of particular importance was recording the IOM care plan and navigation functions in a searchable format. As care paths are developed, risk prediction, palliative care, and other services need to be encompassed. Finally, technology will support the identification and enrollment of eligible patients, and billing activities.
Communications, Revenue Cycle, and Incentives
The final work stream was Communications, Revenue Cycle, and Incentives. For the pilot, the focus was on revenue cycle. A new category of patient needed to be identified, enrolled, and billed to CMS for services. At the outset, the technology did not address the identification of patients receiving only oral drugs. The office visit, the diagnosis, and the drug claim all had to be aligned for enrollment and billing. It was critical to understand the workload by patient and total volume to estimate the technology and personnel needs to meet the initial number of new OCM patients. Communication refers to both internal and external parties. Education of the entire practice staff regarding transformation will be critical for success.
Of the 12 participating practices, 3 practice sites were selected for the pilot program. Each had fewer than 10 medical oncologists and at least 1 radiation oncologist. Each site had a physician champion and an administrative lead. All of the sites were part of a larger regional oncology practice. A fourth site had independently started a pilot and that experience was shared with the larger group as well. The sites were distributed across the country in 4 different time zones.
The pilot experience
The pilot experience yielded important findings, some expected and some unexpected. The challenges of navigation, the treatment plan, and team building were anticipated. We were surprised at the sheer number of potential candidates and the difficulty in finding eligible candidates. Not to be overlooked was a need for continued and possibly increased emphasis on adherence to pathways and process changes to reduce hospitalizations and emergency department (ED) visits.
Navigation
At the outset, none of the pilot practices had formal navigation processes as outlined in Table 1. Many of the processes, such as coordinating appointments and facilitating follow-up services and financial support, were provided by the practice, but were not identified or coordinated as navigation. The practices, as a first step, defined who was responsible for those services and identified 1 person who would be responsible for their completion. It was agreed that navigation was a process shared by a team and not an individual responsibility, yet the person who would monitor the completion of the tasks was not identified. It soon became apparent that true navigation included more tasks than initially outlined.
Additional tasks included appropriate patient education regarding treatment toxicities, follow-up after chemotherapy or a hospitalization, and coordination of other aspects of the IOM care plan, such as survivorship and advance care planning. Each of the practices recruited staff internally to assume the navigator role, and standard operating procedures were developed for completing and documenting this expanded responsibility. True navigation, however, depends on building the team character while still having 1 or 2 members of the team identified as being responsible for following and documenting the patient’s journey through an episode. To meet those needs, navigators developed ad hoc methods, such as spreadsheets, to track patients. The technology team developed drop-down check lists within the EHR, but the burden of documentation continued. Lastly, an ongoing challenge is how best to designate responsibility and assess how many additional staffers are needed.
IOM Care Management Plan
Before initiation of the pilot project, no practice was providing patients with a comprehensive, written treatment plan. Considerably more than half of the members of the work-stream teams believed that would be difficult to implement. However, the members of the Care and Support work stream made some fundamental assumptions to make the care plan workable: first, all aspects of the plan did not occur at the same time, and were not completed by the same person; second, and critically, items 2-9 of Table 2 could be completed at one time during the early conversations between the physician and patient about the goals of treatment. Diagnosis, prognosis, treatment intent, response rate, quality of life, and toxicities were included in the treatment plan, and the remaining IOM care plan could be discussed, or at least identified, as issues for further discussion with other team members. These components were incorporated into a 1-page document that was either typed into the record and printed out or handwritten and copied for the patient. This became the treatment plan and was ready for use at the start of the pilot.
The physician response to the treatment plan was mixed. One site adopted it enthusiastically and quickly moved to use the plan for all patients. Other sites had variable uptake. One hurdle was defining response rate to therapy and prognosis. Data were provided but they often did not match the conditions of individual patients. Some physicians were uncomfortable with the process. Documentation was difficult because the plans had to be scanned into the EHR. Patients generally responded favorably to the plans and would bring them to teaching or chemotherapy sessions.
As with navigation, the treatment plan challenges pointed the technology team toward the development and implementation of an electronic version of the plan. The pilot allowed members of the technology team to visit the clinics, to evaluate workflows and make assumptions on how to structure a treatment plan electronically.
Team-based care
None of the pilot sites had a formalized structure for team-based care. Team huddles were developed and weekly and daily huddles were encouraged. The weekly huddles took about 15-30 minutes, during which patients scheduled for the coming week were reviewed. All personnel who saw patients were invited – benefit counselors, advanced practice providers, schedulers, lab technicians, medical assistants, office and infusion nurses, social workers, pharmacists, physicians, and lead administrative staff. The daily huddle was smaller and generally included a nurse, a medical assistant, and a physician, at a minimum, to review the patients in the hospital, those to be seen in the clinic that day, and any follow-up information based on scheduled contact following recent treatments or events. In some sites, these huddles were uniformly endorsed, in others, not at all. Although many physicians felt that the functions were being handled informally and the additional time commitment would not improve the process, once they began to attend the meetings, they appreciated the value of the huddles and continued attending them. As the complexity of delivery and documentation becomes more apparent, these will prove indispensable to coordinated care.
24/7 access
Hospitalization is one of the chief drivers of the total cost of care, so the pilot sites were concerned that more needed to be done to reduce unnecessary hospitalizations. One site surveyed the patients coming into the clinic about their previous ED visits. Many of the visits had been for noncancer-related events and the clinic was not aware of many of the many of the visits. These findings prompted a number of changes. Open slots were created daily for patients who needed to be worked in for any areas of concern. The on-call physician, triage nurse, or navigation lead could fill these slots. All patients discharged from the hospital were called within 48 hours after discharge and scheduled for a clinic visit within 1 week. Night and weekend call logs were scrutinized each morning and patients’ calls were returned for any issues related to symptom or toxicity management. At one site, patients were given wallet cards with the clinic number, the treatment regimen, and, on the reverse, all symptoms that would justify calling the clinic. The patients were encouraged to call the clinic earlier rather than later in the day. On the back end, the clinics were to have processes in place so that patient calls would be answered quickly to facilitate same-day evaluations in the clinic.
Enrollment and revenue cycle
The most intractable problem was the identification and enrollment of OCM patients. As already noted, 3 components were necessary for enrollment: a drug charge for Part B or D Medicare, a provider visit, and an approved cancer diagnosis. To identify those patients, the claims system would churn out a weekly list of all eligible patients. However, the claims system had no mechanism to pick up Part D claims for oral medications. This meant that any patient with a provider visit and an appropriate diagnosis was potentially eligible for enrollment. At one site, the list of potential patients was 2-3 times the number of actual candidates. It took 6 weeks of manual chart review to resolving the list. Collectively, the 12 practices could have as many as 20,000 patients eligible for the July 1 enrollment. The pilot allowed the practices to get an early start on recruitment of business office staff and plans to address the backlog of potentially eligible patients. The process of identifying eligible patients for the OCM still needs a better solution because finding the appropriate patients is a critical first step in this model.
Underlying all of these initiatives is communication, both internal and external. We have to select and celebrate best practices. We have to educate our staffs. We will have to demonstrate that we are giving better care to our patients by using patient and provider testimonials and data.
From angst to analytics
The challenges of practice transformation can be daunting. It will be difficult to formalize processes and document data in ways that were untested before the pilot program was set up. However, the pilot accomplished 2 things: it identified additional areas that needed improvement and it demonstrated that the most challenging aspects of the OCM were feasible. Navigation and the IOM care plan were broken down into parts; each component was separately addressed, and programs were put in place to make the pieces manageable and part of an overall movement toward team-based care. The addition of a technology platform has been a key factor for the success of the value-based care initiative. Additional technology support has been enlisted to facilitate the processes, and an electronic version of the treatment plan is being tested. More difficult will be efforts to address the cultural resistance to change, which we hope to do by using data and outcomes from the CMS claims data files. The OCM represents an unprecedented opportunity for measurement of the quality of care we deliver.
We are now well und
Acknowledgment
The authors thank Supriya Srinivasan, PhD, for editorial support.
1. Oncology Care Model. Centers for Medicare and Medicaid Services 2016. https://innovation.cms.gov/initiatives/oncology-care/. Last updated November 14, 2017. Accessed November 16 2016.
2. Mortimer L, Strawbridge L, Lukens E, et al. CMS’ Oncology Care Model: delivering higher value cancer care. Clin Pharmacol Ther. 2017.
3. Medicare Prescription Drug, Improvement and Modernization Act of 2003, Pub Law No. 108-173; 2003.
4. Mathews D. The sequester: absolutely everything you could possibly need to know, in one FAQ. https://www.washingtonpost.com/news/wonk/wp/2013/02/20/the-sequester-absolutely-everything-you-could-possibly-need-to-know-in-one-faq/?utm_term=.a0f3a768399b. Published February 20, 2013. Accessed December 4, 2017.
5. Community Oncology Alliance. 2016 community oncology practice impact report: tracking the changing landscape of cancer care. https://www.communityoncology.org/wp-content/uploads/2016/09/PracticeImpactReport-2016-Report.pdf. Issued October 4, 2016. Accessed April 10, 2017.
6. The Patient Protection and Affordable Care Act, Pub Law No. 111-148; 2010.
7. Centers for Medicare and Medicaid Services. Shared Savings Program 2016. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/index.html?redirect=/sharedsavingsprogram/. Last modified October 12, 2017. Accessed November 16 2016.
8. Dale SB, Ghosh A, Peikes DN, et al. Two-year costs and quality in the comprehensive primary care initiative. N Engl J Med. 2016;374:2345-2356.
9. McWilliams JM, Chernew ME, Landon BE, Schwartz AL. Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372:1927-1936.
10. Rajkumar R, Press MJ, Conway PH. The CMS Innovation Center – a five-year self-assessment. N Engl J Med. 2015;372:1981-1983.
11. Waters TM, Webster JA, Stevens LA, et al. Community oncology medical homes: physician-driven change to improve patient care and reduce costs. J Oncol Pract. 2015;11(6):462-467.
12. Wilfong LS, Hoverman JR, Gosse N, Neubauer MA, Torres V. Changing physician compensation and implementing new technology to enhance pathways compliance. J Clin Oncol. 2016;34(Suppl 7S):Abstr 187.
13. Hoverman JR, Cartwright TH, Patt DA, et al. Pathways, outcomes, and costs in colon cancer: retrospective evaluations in two distinct databases. J Oncol Pract. 2011;7(3 Suppl):52s-59s.
14. Neubauer MA, Hoverman JR, Kolodziej M, et al. Cost effectiveness of evidence-based treatment guidelines for the treatment of non-small-cell lung cancer in the community setting. J Oncol Pract. 2010;6(1):12-18.
15. Hoverman JR, Klein I, Harrison DW, et al. Opening the black box: the impact of an oncology management program consisting of level I pathways and an outbound nurse call system. J Oncol Pract. 2014;10(1):63-67.
16. Newcomer LN, Gould B, Page RD, Donelan SA, Perkins M. Changing physician incentives for affordable, quality cancer care: results of an episode payment model. J Oncol Pract. 2014;10(5):322-326.
17. Meyer JA, Rybowski L, Eichler R. Theory and reality of value-based purchasing: lessons from the pioneers. Rockville, MD.: Agency for Health Care Policy and Research; 1997.
18. Stein CM. Academic clinical research: death by a thousand clicks. Sci Transl Med. 2015;7:318fs349.
19. Churchill W. The end of the beginning. 1st ed. Boston, MA: Little Brown & Co; 1943.
In March 2016, 13 practices affiliated with the US Oncology Network (USON) were invited to participate in the Oncology Care Model (OCM) proposed by the Center for Medicare and Medicaid Services (CMS) and Center for Medicare and Medicaid Innovation (CMMI). The OCM, a novel value-based care model, was designed to provide higher-quality and better-coordinated oncology care at a lower cost to CMS.1 Of the 13 practices, 12 agreed to participate with a start date for the program of July 1, 2016. At least 40% of the practices’ patients were insured by Medicare, and any eligible patients with active cancer were offered an opportunity to enter the program. USON practices treat more than 25,000 patients with a qualifying episode per year and the overall OCM program sees more than 150,000 beneficiaries per year,2 so we anticipated that the OCM would have a substantial impact on each of the 12 practices on USON.
Faced with the scenario of having only 3 months between notification of approval and launch of the OCM, it was imperative that all the practices be proactive in planning and preparing to launch the OCM. With this goal in mind, representatives from all OCM candidate practices convened to anticipate the needs of the OCM and chart out a program to meet those needs. In this article, we discuss the requirements and scope of the OCM, the development of an internal pilot project, the anticipated gains from the pilot, and the results and findings from the pilot, both expected and unexpected.
The road to the Oncology Care Model
The government and oncology practices have been on separate trajectories to the OCM. In the last 15 years, the major intersections of these trajectories had to do with price and not patient outcomes. In 2003, the Medicare Prescription Drug Improvement and Modernization Act (MMA) focused on drug price reductions from an average wholesale price–based schedule to an average sales price–based schedule.3 There was the sequester in 2013,4 and more recently a proposal to restructure the payment for Part B drugs. In the background, recurrent negotiations to fix the calculation for the sustainable growth rate allowed for periodic draconian cuts to the prices of services. The cumulative effect of these price reductions has been to put economic pressure on community oncologists such that many have moved to a hospital environment.5
This contentious relationship with community oncology began to change with the passage of the Affordable Care Act (ACA) in 2010.6 Section 3022 of the ACA established the Medicare Shared Savings Program (MSSP) with the charge to create a new type of health care entity that was responsible for achieving the triple aim of improving population health, improving individual patient care, and bending the cost curve.7 Additional programs, such as the Pioneer Accountable Care Organization (ACO) program and the Comprehensive Primary Care Initiative were established to test alternative payment models.8-10
The ACA also funded the CMMI with a mandate to “test innovative payment and service delivery models” to achieve the triple aim; US$10 billion were appropriated for the years 2011-2019 for this purpose. The CMMI funded a pilot project for cancer care, the COME HOME [Community Oncology MEdical HOME] initiative, to test whether some aspects of care could be transformed or augmented to reduce overall costs or at least reduce the rate of increase. Findings from COME HOME have helped inform the OCM program.11
Over the same period, practices belonging to the USON were paving a path toward value. An electronic health record (EHR) for the entire network was adopted in 2005. A pathways program in which chemotherapy regimens were assessed on cost as well as benefits and toxicity, was started in 2006. Higher-cost regimens with no additional benefits comparable with other evidence-based regimens were deselected for initial treatment choices at the time of initial decision support. This process was streamlined using web-based technology that improved pathways compliance and tracking of off-pathways exceptions.12 Retrospective studies indicated that pathways had the potential to bend the cost curve by reducing drug spending.13,14 USON and its practices also tested a nurse call system (Innovent Oncology) funded by a monthly management fee. This program guided patients through chemotherapy with regular telephonic symptom assessment and discussion of patient-centered values and advance care planning. Results of these programs indicated relative reductions in both drug and hospital expenses.15
Additional experience has come from participation in the United Healthcare Episodes of Care (EOC) initiative, which eliminated the chemotherapy drug incentives, compensating physicians on a per-episode basis instead. This study showed a significant reduction in the total cost of cancer therapy after modifying the fee-for-service system and incorporating feedback data and financial incentives to reward improved outcomes and cost efficiency.16
The Oncology Care Model represents a convergence of purchaser demand and provider readiness. The purchaser holds providers accountable for cost and quality. The data on outcomes and costs will provide an extensive database that can be analyzed by the participating practices to address variations and reduce unnecessary care and preventable costs. Best practices are rewarded.17
The OCM and practice readiness
As a part of the CMS proposal process, practices were required to submit implementation plans by June 30, 2015. The purpose of the implementation plan was to define how a practice could transform over 6 broad domains: 24/7 coverage; EHR certification; navigation and care coordination; continuous quality improvement; incorporation of the Institute of Medicine’s (IOM’s) Care Management Plan; and adherence to nationally recognized guidelines. The periods of patient eligibility for the program were 6-month treatment episodes triggered by a cancer diagnosis, a provider encounter claim, and a Part B or D drug claim specifically identified as a cancer treatment. The episodes could be repeated if the 3 criteria continued to be met. All charges continued to be billed as fee-for-service as before, but in addition, participating practices could bill a monthly enhanced oncology services (MEOS) payment for the duration of an episode. Reducing the total cost of care while meeting performance metrics thresholds would also qualify a practice for performance-based payments.
Of the primary components, EHR certification and adherence to guidelines had been addressed previously, but the other domains represented significant challenges. Although 24/7 physician coverage with access to an EHR is standard for all practices, most practice sites do not have an insight into the frequency of hospital admissions, the ability to efficiently add sick patients to the daily schedule, or a routine call system to assess chemotherapy toxicity.
The OCM proposes 10 potential navigation/care coordination functions (Table 1) and does not consider those functions to be the role of one person, but rather a team responsibility. Most of our practices perform at least some of these functions, but they are not formally designated, coordinated, or recorded. A similar condition exists for the IOM care plan, which includes recommendations for treatment and adverse event management (Table 2). The prognosis, toxicity, quality-of-life, and goals-of-care requirements are often found in the physician notes, but not systematically documented or searchable.
Similar challenges have been observed for continuous quality improvement programs. Although the data are available, they are often not easy to search and, therefore, are difficult to retrieve and report. The OCM, as with any transformational program, must always weigh the benefit of information with the burden of consumption of physician and staff time to collect and input these data.18
Prepilot project work
In October 2015, lead physicians and managers from the 12 participating practices were brought together with analytic, technical, process management, and business experts from USON and McKesson Specialty Health. The objective of the meeting was to define the areas of greatest need for day 1 of the OCM and to be prepared. The challenges were to identify the changes needed to meet the requirements of the OCM while improving the patient experience, sustaining the viability of the community oncology practices, creating teams to deliver more effective care, and using data to bend the cost curve. Accordingly, 4 work streams were created: Care and Support; Content; Technology; and Communications, Revenue Cycle and Incentives.
Care and Support
The key tasks of the Care and Support team were to define the workflows for navigation and the IOM care plan. As a patient’s journey through the clinic was mapped out, it became clear that although multiple personnel could participate in the navigation and care plans, there was no systematic way to organize and record the components of successful navigation. The goals for the pilot were to test various options for navigation and to identify best practices that could be translated into standard operating procedures.
Content
The Content team was charged with identifying available programs that would fit into the OCM requirements. These included advance care planning, survivorship, chemotherapy teaching, risk assessment, pathways, and symptom assessment. A longer-term goal was the development of care paths, a more comprehensive map of the patient’s journey that would include consultations, coordinated care, imaging, labs, and other services.
Technology
The task for the Technology work stream was to identify processes of care that required documentation and to evaluate current and future technology solutions to improve efficiencies. The electronic medical record satisfied for the input of data with relevant clinical details, demographics, disease types, and staging. A web-based pathways tool supported clinical decision-making, as well as compliance to pathways. The Medicare quality metric programs set the stage for development of capture and reporting tools for data from many sources. The pilot would indicate the adequacy of these tools and the need for expansion or development of new functions or programs. Of particular importance was recording the IOM care plan and navigation functions in a searchable format. As care paths are developed, risk prediction, palliative care, and other services need to be encompassed. Finally, technology will support the identification and enrollment of eligible patients, and billing activities.
Communications, Revenue Cycle, and Incentives
The final work stream was Communications, Revenue Cycle, and Incentives. For the pilot, the focus was on revenue cycle. A new category of patient needed to be identified, enrolled, and billed to CMS for services. At the outset, the technology did not address the identification of patients receiving only oral drugs. The office visit, the diagnosis, and the drug claim all had to be aligned for enrollment and billing. It was critical to understand the workload by patient and total volume to estimate the technology and personnel needs to meet the initial number of new OCM patients. Communication refers to both internal and external parties. Education of the entire practice staff regarding transformation will be critical for success.
Of the 12 participating practices, 3 practice sites were selected for the pilot program. Each had fewer than 10 medical oncologists and at least 1 radiation oncologist. Each site had a physician champion and an administrative lead. All of the sites were part of a larger regional oncology practice. A fourth site had independently started a pilot and that experience was shared with the larger group as well. The sites were distributed across the country in 4 different time zones.
The pilot experience
The pilot experience yielded important findings, some expected and some unexpected. The challenges of navigation, the treatment plan, and team building were anticipated. We were surprised at the sheer number of potential candidates and the difficulty in finding eligible candidates. Not to be overlooked was a need for continued and possibly increased emphasis on adherence to pathways and process changes to reduce hospitalizations and emergency department (ED) visits.
Navigation
At the outset, none of the pilot practices had formal navigation processes as outlined in Table 1. Many of the processes, such as coordinating appointments and facilitating follow-up services and financial support, were provided by the practice, but were not identified or coordinated as navigation. The practices, as a first step, defined who was responsible for those services and identified 1 person who would be responsible for their completion. It was agreed that navigation was a process shared by a team and not an individual responsibility, yet the person who would monitor the completion of the tasks was not identified. It soon became apparent that true navigation included more tasks than initially outlined.
Additional tasks included appropriate patient education regarding treatment toxicities, follow-up after chemotherapy or a hospitalization, and coordination of other aspects of the IOM care plan, such as survivorship and advance care planning. Each of the practices recruited staff internally to assume the navigator role, and standard operating procedures were developed for completing and documenting this expanded responsibility. True navigation, however, depends on building the team character while still having 1 or 2 members of the team identified as being responsible for following and documenting the patient’s journey through an episode. To meet those needs, navigators developed ad hoc methods, such as spreadsheets, to track patients. The technology team developed drop-down check lists within the EHR, but the burden of documentation continued. Lastly, an ongoing challenge is how best to designate responsibility and assess how many additional staffers are needed.
IOM Care Management Plan
Before initiation of the pilot project, no practice was providing patients with a comprehensive, written treatment plan. Considerably more than half of the members of the work-stream teams believed that would be difficult to implement. However, the members of the Care and Support work stream made some fundamental assumptions to make the care plan workable: first, all aspects of the plan did not occur at the same time, and were not completed by the same person; second, and critically, items 2-9 of Table 2 could be completed at one time during the early conversations between the physician and patient about the goals of treatment. Diagnosis, prognosis, treatment intent, response rate, quality of life, and toxicities were included in the treatment plan, and the remaining IOM care plan could be discussed, or at least identified, as issues for further discussion with other team members. These components were incorporated into a 1-page document that was either typed into the record and printed out or handwritten and copied for the patient. This became the treatment plan and was ready for use at the start of the pilot.
The physician response to the treatment plan was mixed. One site adopted it enthusiastically and quickly moved to use the plan for all patients. Other sites had variable uptake. One hurdle was defining response rate to therapy and prognosis. Data were provided but they often did not match the conditions of individual patients. Some physicians were uncomfortable with the process. Documentation was difficult because the plans had to be scanned into the EHR. Patients generally responded favorably to the plans and would bring them to teaching or chemotherapy sessions.
As with navigation, the treatment plan challenges pointed the technology team toward the development and implementation of an electronic version of the plan. The pilot allowed members of the technology team to visit the clinics, to evaluate workflows and make assumptions on how to structure a treatment plan electronically.
Team-based care
None of the pilot sites had a formalized structure for team-based care. Team huddles were developed and weekly and daily huddles were encouraged. The weekly huddles took about 15-30 minutes, during which patients scheduled for the coming week were reviewed. All personnel who saw patients were invited – benefit counselors, advanced practice providers, schedulers, lab technicians, medical assistants, office and infusion nurses, social workers, pharmacists, physicians, and lead administrative staff. The daily huddle was smaller and generally included a nurse, a medical assistant, and a physician, at a minimum, to review the patients in the hospital, those to be seen in the clinic that day, and any follow-up information based on scheduled contact following recent treatments or events. In some sites, these huddles were uniformly endorsed, in others, not at all. Although many physicians felt that the functions were being handled informally and the additional time commitment would not improve the process, once they began to attend the meetings, they appreciated the value of the huddles and continued attending them. As the complexity of delivery and documentation becomes more apparent, these will prove indispensable to coordinated care.
24/7 access
Hospitalization is one of the chief drivers of the total cost of care, so the pilot sites were concerned that more needed to be done to reduce unnecessary hospitalizations. One site surveyed the patients coming into the clinic about their previous ED visits. Many of the visits had been for noncancer-related events and the clinic was not aware of many of the many of the visits. These findings prompted a number of changes. Open slots were created daily for patients who needed to be worked in for any areas of concern. The on-call physician, triage nurse, or navigation lead could fill these slots. All patients discharged from the hospital were called within 48 hours after discharge and scheduled for a clinic visit within 1 week. Night and weekend call logs were scrutinized each morning and patients’ calls were returned for any issues related to symptom or toxicity management. At one site, patients were given wallet cards with the clinic number, the treatment regimen, and, on the reverse, all symptoms that would justify calling the clinic. The patients were encouraged to call the clinic earlier rather than later in the day. On the back end, the clinics were to have processes in place so that patient calls would be answered quickly to facilitate same-day evaluations in the clinic.
Enrollment and revenue cycle
The most intractable problem was the identification and enrollment of OCM patients. As already noted, 3 components were necessary for enrollment: a drug charge for Part B or D Medicare, a provider visit, and an approved cancer diagnosis. To identify those patients, the claims system would churn out a weekly list of all eligible patients. However, the claims system had no mechanism to pick up Part D claims for oral medications. This meant that any patient with a provider visit and an appropriate diagnosis was potentially eligible for enrollment. At one site, the list of potential patients was 2-3 times the number of actual candidates. It took 6 weeks of manual chart review to resolving the list. Collectively, the 12 practices could have as many as 20,000 patients eligible for the July 1 enrollment. The pilot allowed the practices to get an early start on recruitment of business office staff and plans to address the backlog of potentially eligible patients. The process of identifying eligible patients for the OCM still needs a better solution because finding the appropriate patients is a critical first step in this model.
Underlying all of these initiatives is communication, both internal and external. We have to select and celebrate best practices. We have to educate our staffs. We will have to demonstrate that we are giving better care to our patients by using patient and provider testimonials and data.
From angst to analytics
The challenges of practice transformation can be daunting. It will be difficult to formalize processes and document data in ways that were untested before the pilot program was set up. However, the pilot accomplished 2 things: it identified additional areas that needed improvement and it demonstrated that the most challenging aspects of the OCM were feasible. Navigation and the IOM care plan were broken down into parts; each component was separately addressed, and programs were put in place to make the pieces manageable and part of an overall movement toward team-based care. The addition of a technology platform has been a key factor for the success of the value-based care initiative. Additional technology support has been enlisted to facilitate the processes, and an electronic version of the treatment plan is being tested. More difficult will be efforts to address the cultural resistance to change, which we hope to do by using data and outcomes from the CMS claims data files. The OCM represents an unprecedented opportunity for measurement of the quality of care we deliver.
We are now well und
Acknowledgment
The authors thank Supriya Srinivasan, PhD, for editorial support.
In March 2016, 13 practices affiliated with the US Oncology Network (USON) were invited to participate in the Oncology Care Model (OCM) proposed by the Center for Medicare and Medicaid Services (CMS) and Center for Medicare and Medicaid Innovation (CMMI). The OCM, a novel value-based care model, was designed to provide higher-quality and better-coordinated oncology care at a lower cost to CMS.1 Of the 13 practices, 12 agreed to participate with a start date for the program of July 1, 2016. At least 40% of the practices’ patients were insured by Medicare, and any eligible patients with active cancer were offered an opportunity to enter the program. USON practices treat more than 25,000 patients with a qualifying episode per year and the overall OCM program sees more than 150,000 beneficiaries per year,2 so we anticipated that the OCM would have a substantial impact on each of the 12 practices on USON.
Faced with the scenario of having only 3 months between notification of approval and launch of the OCM, it was imperative that all the practices be proactive in planning and preparing to launch the OCM. With this goal in mind, representatives from all OCM candidate practices convened to anticipate the needs of the OCM and chart out a program to meet those needs. In this article, we discuss the requirements and scope of the OCM, the development of an internal pilot project, the anticipated gains from the pilot, and the results and findings from the pilot, both expected and unexpected.
The road to the Oncology Care Model
The government and oncology practices have been on separate trajectories to the OCM. In the last 15 years, the major intersections of these trajectories had to do with price and not patient outcomes. In 2003, the Medicare Prescription Drug Improvement and Modernization Act (MMA) focused on drug price reductions from an average wholesale price–based schedule to an average sales price–based schedule.3 There was the sequester in 2013,4 and more recently a proposal to restructure the payment for Part B drugs. In the background, recurrent negotiations to fix the calculation for the sustainable growth rate allowed for periodic draconian cuts to the prices of services. The cumulative effect of these price reductions has been to put economic pressure on community oncologists such that many have moved to a hospital environment.5
This contentious relationship with community oncology began to change with the passage of the Affordable Care Act (ACA) in 2010.6 Section 3022 of the ACA established the Medicare Shared Savings Program (MSSP) with the charge to create a new type of health care entity that was responsible for achieving the triple aim of improving population health, improving individual patient care, and bending the cost curve.7 Additional programs, such as the Pioneer Accountable Care Organization (ACO) program and the Comprehensive Primary Care Initiative were established to test alternative payment models.8-10
The ACA also funded the CMMI with a mandate to “test innovative payment and service delivery models” to achieve the triple aim; US$10 billion were appropriated for the years 2011-2019 for this purpose. The CMMI funded a pilot project for cancer care, the COME HOME [Community Oncology MEdical HOME] initiative, to test whether some aspects of care could be transformed or augmented to reduce overall costs or at least reduce the rate of increase. Findings from COME HOME have helped inform the OCM program.11
Over the same period, practices belonging to the USON were paving a path toward value. An electronic health record (EHR) for the entire network was adopted in 2005. A pathways program in which chemotherapy regimens were assessed on cost as well as benefits and toxicity, was started in 2006. Higher-cost regimens with no additional benefits comparable with other evidence-based regimens were deselected for initial treatment choices at the time of initial decision support. This process was streamlined using web-based technology that improved pathways compliance and tracking of off-pathways exceptions.12 Retrospective studies indicated that pathways had the potential to bend the cost curve by reducing drug spending.13,14 USON and its practices also tested a nurse call system (Innovent Oncology) funded by a monthly management fee. This program guided patients through chemotherapy with regular telephonic symptom assessment and discussion of patient-centered values and advance care planning. Results of these programs indicated relative reductions in both drug and hospital expenses.15
Additional experience has come from participation in the United Healthcare Episodes of Care (EOC) initiative, which eliminated the chemotherapy drug incentives, compensating physicians on a per-episode basis instead. This study showed a significant reduction in the total cost of cancer therapy after modifying the fee-for-service system and incorporating feedback data and financial incentives to reward improved outcomes and cost efficiency.16
The Oncology Care Model represents a convergence of purchaser demand and provider readiness. The purchaser holds providers accountable for cost and quality. The data on outcomes and costs will provide an extensive database that can be analyzed by the participating practices to address variations and reduce unnecessary care and preventable costs. Best practices are rewarded.17
The OCM and practice readiness
As a part of the CMS proposal process, practices were required to submit implementation plans by June 30, 2015. The purpose of the implementation plan was to define how a practice could transform over 6 broad domains: 24/7 coverage; EHR certification; navigation and care coordination; continuous quality improvement; incorporation of the Institute of Medicine’s (IOM’s) Care Management Plan; and adherence to nationally recognized guidelines. The periods of patient eligibility for the program were 6-month treatment episodes triggered by a cancer diagnosis, a provider encounter claim, and a Part B or D drug claim specifically identified as a cancer treatment. The episodes could be repeated if the 3 criteria continued to be met. All charges continued to be billed as fee-for-service as before, but in addition, participating practices could bill a monthly enhanced oncology services (MEOS) payment for the duration of an episode. Reducing the total cost of care while meeting performance metrics thresholds would also qualify a practice for performance-based payments.
Of the primary components, EHR certification and adherence to guidelines had been addressed previously, but the other domains represented significant challenges. Although 24/7 physician coverage with access to an EHR is standard for all practices, most practice sites do not have an insight into the frequency of hospital admissions, the ability to efficiently add sick patients to the daily schedule, or a routine call system to assess chemotherapy toxicity.
The OCM proposes 10 potential navigation/care coordination functions (Table 1) and does not consider those functions to be the role of one person, but rather a team responsibility. Most of our practices perform at least some of these functions, but they are not formally designated, coordinated, or recorded. A similar condition exists for the IOM care plan, which includes recommendations for treatment and adverse event management (Table 2). The prognosis, toxicity, quality-of-life, and goals-of-care requirements are often found in the physician notes, but not systematically documented or searchable.
Similar challenges have been observed for continuous quality improvement programs. Although the data are available, they are often not easy to search and, therefore, are difficult to retrieve and report. The OCM, as with any transformational program, must always weigh the benefit of information with the burden of consumption of physician and staff time to collect and input these data.18
Prepilot project work
In October 2015, lead physicians and managers from the 12 participating practices were brought together with analytic, technical, process management, and business experts from USON and McKesson Specialty Health. The objective of the meeting was to define the areas of greatest need for day 1 of the OCM and to be prepared. The challenges were to identify the changes needed to meet the requirements of the OCM while improving the patient experience, sustaining the viability of the community oncology practices, creating teams to deliver more effective care, and using data to bend the cost curve. Accordingly, 4 work streams were created: Care and Support; Content; Technology; and Communications, Revenue Cycle and Incentives.
Care and Support
The key tasks of the Care and Support team were to define the workflows for navigation and the IOM care plan. As a patient’s journey through the clinic was mapped out, it became clear that although multiple personnel could participate in the navigation and care plans, there was no systematic way to organize and record the components of successful navigation. The goals for the pilot were to test various options for navigation and to identify best practices that could be translated into standard operating procedures.
Content
The Content team was charged with identifying available programs that would fit into the OCM requirements. These included advance care planning, survivorship, chemotherapy teaching, risk assessment, pathways, and symptom assessment. A longer-term goal was the development of care paths, a more comprehensive map of the patient’s journey that would include consultations, coordinated care, imaging, labs, and other services.
Technology
The task for the Technology work stream was to identify processes of care that required documentation and to evaluate current and future technology solutions to improve efficiencies. The electronic medical record satisfied for the input of data with relevant clinical details, demographics, disease types, and staging. A web-based pathways tool supported clinical decision-making, as well as compliance to pathways. The Medicare quality metric programs set the stage for development of capture and reporting tools for data from many sources. The pilot would indicate the adequacy of these tools and the need for expansion or development of new functions or programs. Of particular importance was recording the IOM care plan and navigation functions in a searchable format. As care paths are developed, risk prediction, palliative care, and other services need to be encompassed. Finally, technology will support the identification and enrollment of eligible patients, and billing activities.
Communications, Revenue Cycle, and Incentives
The final work stream was Communications, Revenue Cycle, and Incentives. For the pilot, the focus was on revenue cycle. A new category of patient needed to be identified, enrolled, and billed to CMS for services. At the outset, the technology did not address the identification of patients receiving only oral drugs. The office visit, the diagnosis, and the drug claim all had to be aligned for enrollment and billing. It was critical to understand the workload by patient and total volume to estimate the technology and personnel needs to meet the initial number of new OCM patients. Communication refers to both internal and external parties. Education of the entire practice staff regarding transformation will be critical for success.
Of the 12 participating practices, 3 practice sites were selected for the pilot program. Each had fewer than 10 medical oncologists and at least 1 radiation oncologist. Each site had a physician champion and an administrative lead. All of the sites were part of a larger regional oncology practice. A fourth site had independently started a pilot and that experience was shared with the larger group as well. The sites were distributed across the country in 4 different time zones.
The pilot experience
The pilot experience yielded important findings, some expected and some unexpected. The challenges of navigation, the treatment plan, and team building were anticipated. We were surprised at the sheer number of potential candidates and the difficulty in finding eligible candidates. Not to be overlooked was a need for continued and possibly increased emphasis on adherence to pathways and process changes to reduce hospitalizations and emergency department (ED) visits.
Navigation
At the outset, none of the pilot practices had formal navigation processes as outlined in Table 1. Many of the processes, such as coordinating appointments and facilitating follow-up services and financial support, were provided by the practice, but were not identified or coordinated as navigation. The practices, as a first step, defined who was responsible for those services and identified 1 person who would be responsible for their completion. It was agreed that navigation was a process shared by a team and not an individual responsibility, yet the person who would monitor the completion of the tasks was not identified. It soon became apparent that true navigation included more tasks than initially outlined.
Additional tasks included appropriate patient education regarding treatment toxicities, follow-up after chemotherapy or a hospitalization, and coordination of other aspects of the IOM care plan, such as survivorship and advance care planning. Each of the practices recruited staff internally to assume the navigator role, and standard operating procedures were developed for completing and documenting this expanded responsibility. True navigation, however, depends on building the team character while still having 1 or 2 members of the team identified as being responsible for following and documenting the patient’s journey through an episode. To meet those needs, navigators developed ad hoc methods, such as spreadsheets, to track patients. The technology team developed drop-down check lists within the EHR, but the burden of documentation continued. Lastly, an ongoing challenge is how best to designate responsibility and assess how many additional staffers are needed.
IOM Care Management Plan
Before initiation of the pilot project, no practice was providing patients with a comprehensive, written treatment plan. Considerably more than half of the members of the work-stream teams believed that would be difficult to implement. However, the members of the Care and Support work stream made some fundamental assumptions to make the care plan workable: first, all aspects of the plan did not occur at the same time, and were not completed by the same person; second, and critically, items 2-9 of Table 2 could be completed at one time during the early conversations between the physician and patient about the goals of treatment. Diagnosis, prognosis, treatment intent, response rate, quality of life, and toxicities were included in the treatment plan, and the remaining IOM care plan could be discussed, or at least identified, as issues for further discussion with other team members. These components were incorporated into a 1-page document that was either typed into the record and printed out or handwritten and copied for the patient. This became the treatment plan and was ready for use at the start of the pilot.
The physician response to the treatment plan was mixed. One site adopted it enthusiastically and quickly moved to use the plan for all patients. Other sites had variable uptake. One hurdle was defining response rate to therapy and prognosis. Data were provided but they often did not match the conditions of individual patients. Some physicians were uncomfortable with the process. Documentation was difficult because the plans had to be scanned into the EHR. Patients generally responded favorably to the plans and would bring them to teaching or chemotherapy sessions.
As with navigation, the treatment plan challenges pointed the technology team toward the development and implementation of an electronic version of the plan. The pilot allowed members of the technology team to visit the clinics, to evaluate workflows and make assumptions on how to structure a treatment plan electronically.
Team-based care
None of the pilot sites had a formalized structure for team-based care. Team huddles were developed and weekly and daily huddles were encouraged. The weekly huddles took about 15-30 minutes, during which patients scheduled for the coming week were reviewed. All personnel who saw patients were invited – benefit counselors, advanced practice providers, schedulers, lab technicians, medical assistants, office and infusion nurses, social workers, pharmacists, physicians, and lead administrative staff. The daily huddle was smaller and generally included a nurse, a medical assistant, and a physician, at a minimum, to review the patients in the hospital, those to be seen in the clinic that day, and any follow-up information based on scheduled contact following recent treatments or events. In some sites, these huddles were uniformly endorsed, in others, not at all. Although many physicians felt that the functions were being handled informally and the additional time commitment would not improve the process, once they began to attend the meetings, they appreciated the value of the huddles and continued attending them. As the complexity of delivery and documentation becomes more apparent, these will prove indispensable to coordinated care.
24/7 access
Hospitalization is one of the chief drivers of the total cost of care, so the pilot sites were concerned that more needed to be done to reduce unnecessary hospitalizations. One site surveyed the patients coming into the clinic about their previous ED visits. Many of the visits had been for noncancer-related events and the clinic was not aware of many of the many of the visits. These findings prompted a number of changes. Open slots were created daily for patients who needed to be worked in for any areas of concern. The on-call physician, triage nurse, or navigation lead could fill these slots. All patients discharged from the hospital were called within 48 hours after discharge and scheduled for a clinic visit within 1 week. Night and weekend call logs were scrutinized each morning and patients’ calls were returned for any issues related to symptom or toxicity management. At one site, patients were given wallet cards with the clinic number, the treatment regimen, and, on the reverse, all symptoms that would justify calling the clinic. The patients were encouraged to call the clinic earlier rather than later in the day. On the back end, the clinics were to have processes in place so that patient calls would be answered quickly to facilitate same-day evaluations in the clinic.
Enrollment and revenue cycle
The most intractable problem was the identification and enrollment of OCM patients. As already noted, 3 components were necessary for enrollment: a drug charge for Part B or D Medicare, a provider visit, and an approved cancer diagnosis. To identify those patients, the claims system would churn out a weekly list of all eligible patients. However, the claims system had no mechanism to pick up Part D claims for oral medications. This meant that any patient with a provider visit and an appropriate diagnosis was potentially eligible for enrollment. At one site, the list of potential patients was 2-3 times the number of actual candidates. It took 6 weeks of manual chart review to resolving the list. Collectively, the 12 practices could have as many as 20,000 patients eligible for the July 1 enrollment. The pilot allowed the practices to get an early start on recruitment of business office staff and plans to address the backlog of potentially eligible patients. The process of identifying eligible patients for the OCM still needs a better solution because finding the appropriate patients is a critical first step in this model.
Underlying all of these initiatives is communication, both internal and external. We have to select and celebrate best practices. We have to educate our staffs. We will have to demonstrate that we are giving better care to our patients by using patient and provider testimonials and data.
From angst to analytics
The challenges of practice transformation can be daunting. It will be difficult to formalize processes and document data in ways that were untested before the pilot program was set up. However, the pilot accomplished 2 things: it identified additional areas that needed improvement and it demonstrated that the most challenging aspects of the OCM were feasible. Navigation and the IOM care plan were broken down into parts; each component was separately addressed, and programs were put in place to make the pieces manageable and part of an overall movement toward team-based care. The addition of a technology platform has been a key factor for the success of the value-based care initiative. Additional technology support has been enlisted to facilitate the processes, and an electronic version of the treatment plan is being tested. More difficult will be efforts to address the cultural resistance to change, which we hope to do by using data and outcomes from the CMS claims data files. The OCM represents an unprecedented opportunity for measurement of the quality of care we deliver.
We are now well und
Acknowledgment
The authors thank Supriya Srinivasan, PhD, for editorial support.
1. Oncology Care Model. Centers for Medicare and Medicaid Services 2016. https://innovation.cms.gov/initiatives/oncology-care/. Last updated November 14, 2017. Accessed November 16 2016.
2. Mortimer L, Strawbridge L, Lukens E, et al. CMS’ Oncology Care Model: delivering higher value cancer care. Clin Pharmacol Ther. 2017.
3. Medicare Prescription Drug, Improvement and Modernization Act of 2003, Pub Law No. 108-173; 2003.
4. Mathews D. The sequester: absolutely everything you could possibly need to know, in one FAQ. https://www.washingtonpost.com/news/wonk/wp/2013/02/20/the-sequester-absolutely-everything-you-could-possibly-need-to-know-in-one-faq/?utm_term=.a0f3a768399b. Published February 20, 2013. Accessed December 4, 2017.
5. Community Oncology Alliance. 2016 community oncology practice impact report: tracking the changing landscape of cancer care. https://www.communityoncology.org/wp-content/uploads/2016/09/PracticeImpactReport-2016-Report.pdf. Issued October 4, 2016. Accessed April 10, 2017.
6. The Patient Protection and Affordable Care Act, Pub Law No. 111-148; 2010.
7. Centers for Medicare and Medicaid Services. Shared Savings Program 2016. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/index.html?redirect=/sharedsavingsprogram/. Last modified October 12, 2017. Accessed November 16 2016.
8. Dale SB, Ghosh A, Peikes DN, et al. Two-year costs and quality in the comprehensive primary care initiative. N Engl J Med. 2016;374:2345-2356.
9. McWilliams JM, Chernew ME, Landon BE, Schwartz AL. Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372:1927-1936.
10. Rajkumar R, Press MJ, Conway PH. The CMS Innovation Center – a five-year self-assessment. N Engl J Med. 2015;372:1981-1983.
11. Waters TM, Webster JA, Stevens LA, et al. Community oncology medical homes: physician-driven change to improve patient care and reduce costs. J Oncol Pract. 2015;11(6):462-467.
12. Wilfong LS, Hoverman JR, Gosse N, Neubauer MA, Torres V. Changing physician compensation and implementing new technology to enhance pathways compliance. J Clin Oncol. 2016;34(Suppl 7S):Abstr 187.
13. Hoverman JR, Cartwright TH, Patt DA, et al. Pathways, outcomes, and costs in colon cancer: retrospective evaluations in two distinct databases. J Oncol Pract. 2011;7(3 Suppl):52s-59s.
14. Neubauer MA, Hoverman JR, Kolodziej M, et al. Cost effectiveness of evidence-based treatment guidelines for the treatment of non-small-cell lung cancer in the community setting. J Oncol Pract. 2010;6(1):12-18.
15. Hoverman JR, Klein I, Harrison DW, et al. Opening the black box: the impact of an oncology management program consisting of level I pathways and an outbound nurse call system. J Oncol Pract. 2014;10(1):63-67.
16. Newcomer LN, Gould B, Page RD, Donelan SA, Perkins M. Changing physician incentives for affordable, quality cancer care: results of an episode payment model. J Oncol Pract. 2014;10(5):322-326.
17. Meyer JA, Rybowski L, Eichler R. Theory and reality of value-based purchasing: lessons from the pioneers. Rockville, MD.: Agency for Health Care Policy and Research; 1997.
18. Stein CM. Academic clinical research: death by a thousand clicks. Sci Transl Med. 2015;7:318fs349.
19. Churchill W. The end of the beginning. 1st ed. Boston, MA: Little Brown & Co; 1943.
1. Oncology Care Model. Centers for Medicare and Medicaid Services 2016. https://innovation.cms.gov/initiatives/oncology-care/. Last updated November 14, 2017. Accessed November 16 2016.
2. Mortimer L, Strawbridge L, Lukens E, et al. CMS’ Oncology Care Model: delivering higher value cancer care. Clin Pharmacol Ther. 2017.
3. Medicare Prescription Drug, Improvement and Modernization Act of 2003, Pub Law No. 108-173; 2003.
4. Mathews D. The sequester: absolutely everything you could possibly need to know, in one FAQ. https://www.washingtonpost.com/news/wonk/wp/2013/02/20/the-sequester-absolutely-everything-you-could-possibly-need-to-know-in-one-faq/?utm_term=.a0f3a768399b. Published February 20, 2013. Accessed December 4, 2017.
5. Community Oncology Alliance. 2016 community oncology practice impact report: tracking the changing landscape of cancer care. https://www.communityoncology.org/wp-content/uploads/2016/09/PracticeImpactReport-2016-Report.pdf. Issued October 4, 2016. Accessed April 10, 2017.
6. The Patient Protection and Affordable Care Act, Pub Law No. 111-148; 2010.
7. Centers for Medicare and Medicaid Services. Shared Savings Program 2016. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/index.html?redirect=/sharedsavingsprogram/. Last modified October 12, 2017. Accessed November 16 2016.
8. Dale SB, Ghosh A, Peikes DN, et al. Two-year costs and quality in the comprehensive primary care initiative. N Engl J Med. 2016;374:2345-2356.
9. McWilliams JM, Chernew ME, Landon BE, Schwartz AL. Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372:1927-1936.
10. Rajkumar R, Press MJ, Conway PH. The CMS Innovation Center – a five-year self-assessment. N Engl J Med. 2015;372:1981-1983.
11. Waters TM, Webster JA, Stevens LA, et al. Community oncology medical homes: physician-driven change to improve patient care and reduce costs. J Oncol Pract. 2015;11(6):462-467.
12. Wilfong LS, Hoverman JR, Gosse N, Neubauer MA, Torres V. Changing physician compensation and implementing new technology to enhance pathways compliance. J Clin Oncol. 2016;34(Suppl 7S):Abstr 187.
13. Hoverman JR, Cartwright TH, Patt DA, et al. Pathways, outcomes, and costs in colon cancer: retrospective evaluations in two distinct databases. J Oncol Pract. 2011;7(3 Suppl):52s-59s.
14. Neubauer MA, Hoverman JR, Kolodziej M, et al. Cost effectiveness of evidence-based treatment guidelines for the treatment of non-small-cell lung cancer in the community setting. J Oncol Pract. 2010;6(1):12-18.
15. Hoverman JR, Klein I, Harrison DW, et al. Opening the black box: the impact of an oncology management program consisting of level I pathways and an outbound nurse call system. J Oncol Pract. 2014;10(1):63-67.
16. Newcomer LN, Gould B, Page RD, Donelan SA, Perkins M. Changing physician incentives for affordable, quality cancer care: results of an episode payment model. J Oncol Pract. 2014;10(5):322-326.
17. Meyer JA, Rybowski L, Eichler R. Theory and reality of value-based purchasing: lessons from the pioneers. Rockville, MD.: Agency for Health Care Policy and Research; 1997.
18. Stein CM. Academic clinical research: death by a thousand clicks. Sci Transl Med. 2015;7:318fs349.
19. Churchill W. The end of the beginning. 1st ed. Boston, MA: Little Brown & Co; 1943.