User login
For patients with extensive mild to moderate ulcerative colitis, numerous randomized controlled trials support the use of either standard-dose mesalamine (2-3 grams per day) or diazo-bonded 5-aminosalicylic acid (ASA) instead of low-dose mesalamine, sulfasalazine, or no therapy, state new guidelines from the American Gastroenterological Association, published in Gastroenterology.
Sulfasalazine (2-4 grams per day) is less likely to be tolerated but remains a “reasonable option” for remitted patients who are already on it and for patients with prominent arthritis symptoms, especially if alternative treatments are cost prohibitive, wrote Cynthia W. Ko, MD, MS, of the University of Washington, Seattle, and her associates.
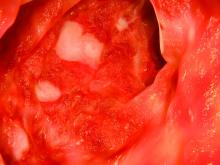
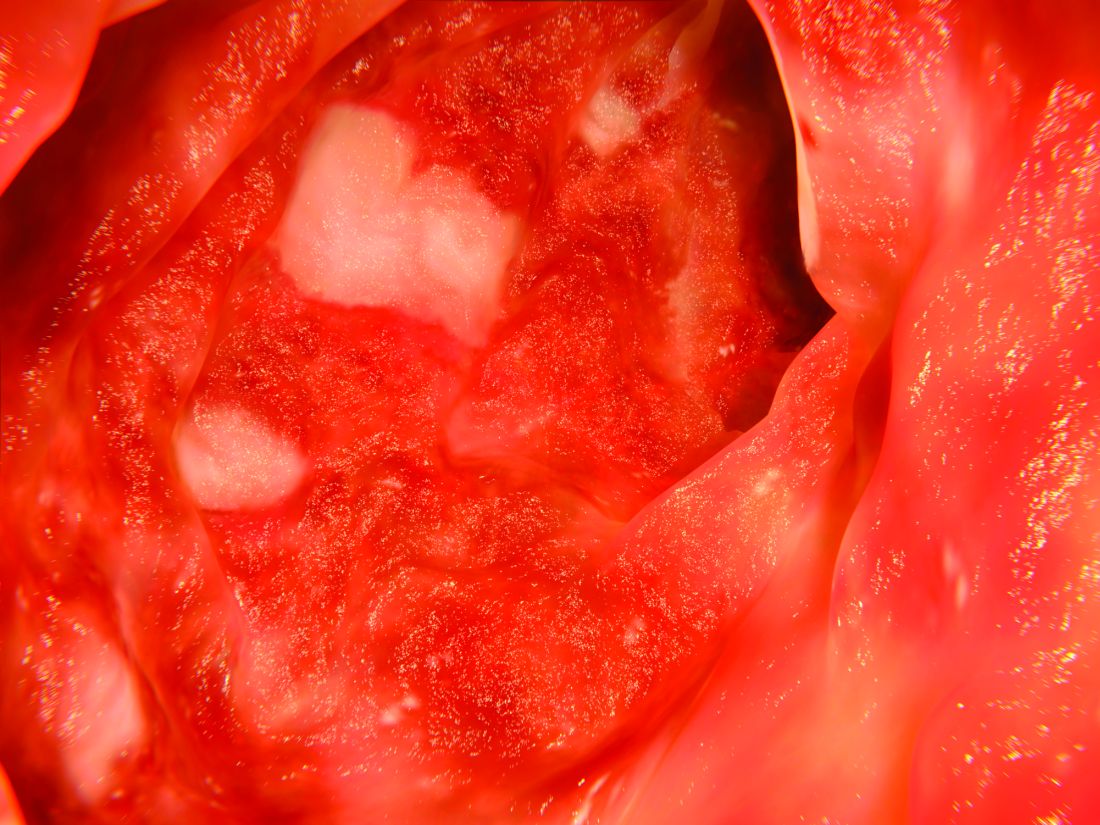
According to the guideline, patients with mild to moderate ulcerative colitis have less than four to six bowel movements per day, only mild or moderate rectal bleeding, no constitutional symptoms, and no high overall inflammatory burden or signs of high inflammatory activity on the Mayo Clinic score and Truelove and Witt’s criteria. These patients usually do not require colectomy, but this outcome is more likely when patients are diagnosed before age 40 years or have extensive disease or deep ulcers, extraintestinal manifestations, or elevated inflammatory markers. These higher-risk patients need more aggressive initial treatment and faster treatment intensification in cases of inadequate response, the guideline emphasizes. Even for cases of mild to moderate ulcerative colitis, treatment intensification is preferable to repeated courses of corticosteroids.
The guideline recommends adding rectal mesalamine to oral 5-ASA if patients have extensive or left-sided mild to moderate ulcerative colitis. In randomized controlled trials, this combination was significantly more likely to induce and maintain remission than was standard-dose oral mesalamine monotherapy, the authors noted. “In the maintenance trials, enemas were used twice per week or for 1 week per month. Both oral and topical mesalamine were well tolerated.”
For patients with moderate disease activity or a suboptimal response to standard-dose mesalamine or diazo-bonded 5-ASA, the guideline recommends adding rectal mesalamine to high-dose oral mesalamine (more than 3 grams daily). Combination therapy maximizes the delivery of mesalamine to the affected area of the colon, which optimizes the trial of 5-ASA before opting for treatment escalation, the authors noted. They recommend once-daily oral mesalamine dosing, since this is easier to adhere to and studies have found no benefit of more frequent dosing.
For inducing remission of mild to moderate ulcerative colitis, the guideline recommends standard-dose oral mesalamine or diazo-bonded 5-ASA over budesonide. “Overall, the budesonide preparations are not superior to mesalamine for induction of remission,” the authors wrote. Oral 5-ASAs are preferred, especially given the absence of data on the efficacy or safety of maintenance budesonide therapy.
For patients with mild to moderate ulcerative proctosigmoiditis or proctitis, the guideline conditionally recommends rectal mesalamine over oral mesalamine. Compared with placebo, rectal mesalamine suppositories were significantly more likely to induce remission in randomized trials of patients with mild to moderate ulcerative proctitis. If these patients cannot tolerate or are refractory to mesalamine suppositories, low-quality evidence supports rectal steroid therapy over no treatment, the guideline states. For patients with mild to moderate ulcerative proctosigmoiditis, moderate-quality evidence supports mesalamine enemas over rectal corticosteroids. If these patients want to avoid the difficulties of enemas, the guideline considers rectal corticosteroid foam a reasonable alternative.
Likewise, they cite low-quality evidence for adding oral prednisone or budesonide MMX to 5-ASA if patients are refractory to optimized 5-ASA therapy. No trials have directly compared rates of remission with budesonide MMX versus systemic corticosteroids. In just one placebo-controlled trial, adding budesonide MMX to 5-ASA slightly improved the chances of remission (risk ratio, 0.95; 95% confidence interval, 0.89-1.00). Furthermore, studies of other second-generation corticosteroids found they were better tolerated but no more likely to induce remission than oral prednisone or prednisolone.
Some patients with mild to moderate colitis respond inadequately to these recommended therapies and need systemic corticosteroids, immunomodulators, or biologic therapies to induce and maintain remission, the guideline authors noted. They make no recommendation on immunomodulators or biologics. Studies of probiotics, curcumin, and fecal microbiota transplantation are “urgently needed,” but for now, their use “risks delaying proven effective therapy, with the potential for worsening symptoms or complications,” they wrote. For patients without Clostridium difficile infections, they recommend against fecal microbiota transplantation except in the setting of a clinical trial.
The experts also noted the need for a tool to stratify patients with mild to moderate ulcerative colitis based on their risk of future progression and colectomy.
Finally, they call for studies on who will benefit most from high-dose mesalamine or topical mesalamine and on the relative safety and efficacy of budesonide and systemic corticosteroids in the event of an inadequate response to 5-ASAs.
All members were required to complete the disclosure statement. These statements are maintained at the American Gastroenterological Association headquarters in Bethesda, Maryland, and pertinent disclosures of conflict of interest are published with this report.
SOURCE: Crocket SD et al. Gastro 2019;156(2). doi: org/10.1053/j.gastro.2018.12.009.
For patients with extensive mild to moderate ulcerative colitis, numerous randomized controlled trials support the use of either standard-dose mesalamine (2-3 grams per day) or diazo-bonded 5-aminosalicylic acid (ASA) instead of low-dose mesalamine, sulfasalazine, or no therapy, state new guidelines from the American Gastroenterological Association, published in Gastroenterology.
Sulfasalazine (2-4 grams per day) is less likely to be tolerated but remains a “reasonable option” for remitted patients who are already on it and for patients with prominent arthritis symptoms, especially if alternative treatments are cost prohibitive, wrote Cynthia W. Ko, MD, MS, of the University of Washington, Seattle, and her associates.
According to the guideline, patients with mild to moderate ulcerative colitis have less than four to six bowel movements per day, only mild or moderate rectal bleeding, no constitutional symptoms, and no high overall inflammatory burden or signs of high inflammatory activity on the Mayo Clinic score and Truelove and Witt’s criteria. These patients usually do not require colectomy, but this outcome is more likely when patients are diagnosed before age 40 years or have extensive disease or deep ulcers, extraintestinal manifestations, or elevated inflammatory markers. These higher-risk patients need more aggressive initial treatment and faster treatment intensification in cases of inadequate response, the guideline emphasizes. Even for cases of mild to moderate ulcerative colitis, treatment intensification is preferable to repeated courses of corticosteroids.
The guideline recommends adding rectal mesalamine to oral 5-ASA if patients have extensive or left-sided mild to moderate ulcerative colitis. In randomized controlled trials, this combination was significantly more likely to induce and maintain remission than was standard-dose oral mesalamine monotherapy, the authors noted. “In the maintenance trials, enemas were used twice per week or for 1 week per month. Both oral and topical mesalamine were well tolerated.”
For patients with moderate disease activity or a suboptimal response to standard-dose mesalamine or diazo-bonded 5-ASA, the guideline recommends adding rectal mesalamine to high-dose oral mesalamine (more than 3 grams daily). Combination therapy maximizes the delivery of mesalamine to the affected area of the colon, which optimizes the trial of 5-ASA before opting for treatment escalation, the authors noted. They recommend once-daily oral mesalamine dosing, since this is easier to adhere to and studies have found no benefit of more frequent dosing.
For inducing remission of mild to moderate ulcerative colitis, the guideline recommends standard-dose oral mesalamine or diazo-bonded 5-ASA over budesonide. “Overall, the budesonide preparations are not superior to mesalamine for induction of remission,” the authors wrote. Oral 5-ASAs are preferred, especially given the absence of data on the efficacy or safety of maintenance budesonide therapy.
For patients with mild to moderate ulcerative proctosigmoiditis or proctitis, the guideline conditionally recommends rectal mesalamine over oral mesalamine. Compared with placebo, rectal mesalamine suppositories were significantly more likely to induce remission in randomized trials of patients with mild to moderate ulcerative proctitis. If these patients cannot tolerate or are refractory to mesalamine suppositories, low-quality evidence supports rectal steroid therapy over no treatment, the guideline states. For patients with mild to moderate ulcerative proctosigmoiditis, moderate-quality evidence supports mesalamine enemas over rectal corticosteroids. If these patients want to avoid the difficulties of enemas, the guideline considers rectal corticosteroid foam a reasonable alternative.
Likewise, they cite low-quality evidence for adding oral prednisone or budesonide MMX to 5-ASA if patients are refractory to optimized 5-ASA therapy. No trials have directly compared rates of remission with budesonide MMX versus systemic corticosteroids. In just one placebo-controlled trial, adding budesonide MMX to 5-ASA slightly improved the chances of remission (risk ratio, 0.95; 95% confidence interval, 0.89-1.00). Furthermore, studies of other second-generation corticosteroids found they were better tolerated but no more likely to induce remission than oral prednisone or prednisolone.
Some patients with mild to moderate colitis respond inadequately to these recommended therapies and need systemic corticosteroids, immunomodulators, or biologic therapies to induce and maintain remission, the guideline authors noted. They make no recommendation on immunomodulators or biologics. Studies of probiotics, curcumin, and fecal microbiota transplantation are “urgently needed,” but for now, their use “risks delaying proven effective therapy, with the potential for worsening symptoms or complications,” they wrote. For patients without Clostridium difficile infections, they recommend against fecal microbiota transplantation except in the setting of a clinical trial.
The experts also noted the need for a tool to stratify patients with mild to moderate ulcerative colitis based on their risk of future progression and colectomy.
Finally, they call for studies on who will benefit most from high-dose mesalamine or topical mesalamine and on the relative safety and efficacy of budesonide and systemic corticosteroids in the event of an inadequate response to 5-ASAs.
All members were required to complete the disclosure statement. These statements are maintained at the American Gastroenterological Association headquarters in Bethesda, Maryland, and pertinent disclosures of conflict of interest are published with this report.
SOURCE: Crocket SD et al. Gastro 2019;156(2). doi: org/10.1053/j.gastro.2018.12.009.
For patients with extensive mild to moderate ulcerative colitis, numerous randomized controlled trials support the use of either standard-dose mesalamine (2-3 grams per day) or diazo-bonded 5-aminosalicylic acid (ASA) instead of low-dose mesalamine, sulfasalazine, or no therapy, state new guidelines from the American Gastroenterological Association, published in Gastroenterology.
Sulfasalazine (2-4 grams per day) is less likely to be tolerated but remains a “reasonable option” for remitted patients who are already on it and for patients with prominent arthritis symptoms, especially if alternative treatments are cost prohibitive, wrote Cynthia W. Ko, MD, MS, of the University of Washington, Seattle, and her associates.
According to the guideline, patients with mild to moderate ulcerative colitis have less than four to six bowel movements per day, only mild or moderate rectal bleeding, no constitutional symptoms, and no high overall inflammatory burden or signs of high inflammatory activity on the Mayo Clinic score and Truelove and Witt’s criteria. These patients usually do not require colectomy, but this outcome is more likely when patients are diagnosed before age 40 years or have extensive disease or deep ulcers, extraintestinal manifestations, or elevated inflammatory markers. These higher-risk patients need more aggressive initial treatment and faster treatment intensification in cases of inadequate response, the guideline emphasizes. Even for cases of mild to moderate ulcerative colitis, treatment intensification is preferable to repeated courses of corticosteroids.
The guideline recommends adding rectal mesalamine to oral 5-ASA if patients have extensive or left-sided mild to moderate ulcerative colitis. In randomized controlled trials, this combination was significantly more likely to induce and maintain remission than was standard-dose oral mesalamine monotherapy, the authors noted. “In the maintenance trials, enemas were used twice per week or for 1 week per month. Both oral and topical mesalamine were well tolerated.”
For patients with moderate disease activity or a suboptimal response to standard-dose mesalamine or diazo-bonded 5-ASA, the guideline recommends adding rectal mesalamine to high-dose oral mesalamine (more than 3 grams daily). Combination therapy maximizes the delivery of mesalamine to the affected area of the colon, which optimizes the trial of 5-ASA before opting for treatment escalation, the authors noted. They recommend once-daily oral mesalamine dosing, since this is easier to adhere to and studies have found no benefit of more frequent dosing.
For inducing remission of mild to moderate ulcerative colitis, the guideline recommends standard-dose oral mesalamine or diazo-bonded 5-ASA over budesonide. “Overall, the budesonide preparations are not superior to mesalamine for induction of remission,” the authors wrote. Oral 5-ASAs are preferred, especially given the absence of data on the efficacy or safety of maintenance budesonide therapy.
For patients with mild to moderate ulcerative proctosigmoiditis or proctitis, the guideline conditionally recommends rectal mesalamine over oral mesalamine. Compared with placebo, rectal mesalamine suppositories were significantly more likely to induce remission in randomized trials of patients with mild to moderate ulcerative proctitis. If these patients cannot tolerate or are refractory to mesalamine suppositories, low-quality evidence supports rectal steroid therapy over no treatment, the guideline states. For patients with mild to moderate ulcerative proctosigmoiditis, moderate-quality evidence supports mesalamine enemas over rectal corticosteroids. If these patients want to avoid the difficulties of enemas, the guideline considers rectal corticosteroid foam a reasonable alternative.
Likewise, they cite low-quality evidence for adding oral prednisone or budesonide MMX to 5-ASA if patients are refractory to optimized 5-ASA therapy. No trials have directly compared rates of remission with budesonide MMX versus systemic corticosteroids. In just one placebo-controlled trial, adding budesonide MMX to 5-ASA slightly improved the chances of remission (risk ratio, 0.95; 95% confidence interval, 0.89-1.00). Furthermore, studies of other second-generation corticosteroids found they were better tolerated but no more likely to induce remission than oral prednisone or prednisolone.
Some patients with mild to moderate colitis respond inadequately to these recommended therapies and need systemic corticosteroids, immunomodulators, or biologic therapies to induce and maintain remission, the guideline authors noted. They make no recommendation on immunomodulators or biologics. Studies of probiotics, curcumin, and fecal microbiota transplantation are “urgently needed,” but for now, their use “risks delaying proven effective therapy, with the potential for worsening symptoms or complications,” they wrote. For patients without Clostridium difficile infections, they recommend against fecal microbiota transplantation except in the setting of a clinical trial.
The experts also noted the need for a tool to stratify patients with mild to moderate ulcerative colitis based on their risk of future progression and colectomy.
Finally, they call for studies on who will benefit most from high-dose mesalamine or topical mesalamine and on the relative safety and efficacy of budesonide and systemic corticosteroids in the event of an inadequate response to 5-ASAs.
All members were required to complete the disclosure statement. These statements are maintained at the American Gastroenterological Association headquarters in Bethesda, Maryland, and pertinent disclosures of conflict of interest are published with this report.
SOURCE: Crocket SD et al. Gastro 2019;156(2). doi: org/10.1053/j.gastro.2018.12.009.
FROM GASTROENTEROLOGY