User login
Acute generalized exanthematous pustulosis (AGEP) is a potentially widespread, pustular, cutaneous eruption. In 90% of cases, AGEP results from drug administration.1,2 It manifests as numerous subcorneal, nonfollicular, sterile pustules of rapid onset on an erythematous base,2 often in conjunction with fever, peripheral leukocytosis, and neutrophilia.3 Numerous drug therapies have been implicated in the etiology of AGEP, most commonly the β-lactam antibiotics, such as the penicillin derivatives and cephalosporins.2 Typically, AGEP occurs soon after drug ingestion and resolves spontaneously, shortly after the causative drug is discontinued.
Ranolazine is an antianginal, anti-ischemic medication with an undetermined mechanism of action. Its antianginal and anti-ischemic effects do not depend on reduced heart rate or blood pressure. At therapeutic levels, it inhibits the cardiac late sodium current (INa), reducing the sodium-induced calcium overload in ischemic cardiac myocytes. Severe adverse reactions include angioedema; paresthesia; pancytopenia; and, in animal studies, tumorigenicity.4 Herein we report a case of AGEP associated with the use of ranolazine.
Case Report
An 83-year-old man presented with a generalized rash of approximately 12 days’ duration. The patient reported that the small “pimple-like” bumps initially erupted on the back of the neck but gradually spread to the chest, back, and extremities. The lesions were asymptomatic at the outset and became pruritic over time. For the last several years, the patient had been taking tamsulosin for benign prostatic hypertrophy and rosuvastatin for hyperlipidemia. Twelve days prior to the exanthem, he had started taking ranolazine for symptomatic ischemia until coronary angiography could be performed. He reported having no associated fevers, chills, or malaise and had no personal history of psoriasis, though he had a maternal history of the disorder.
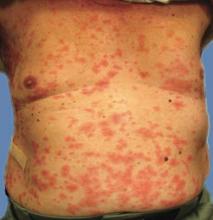
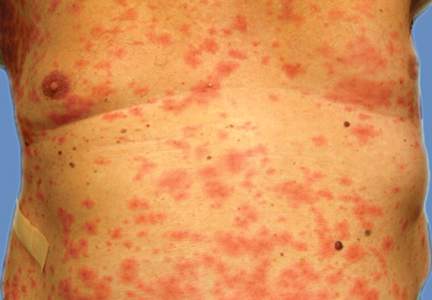
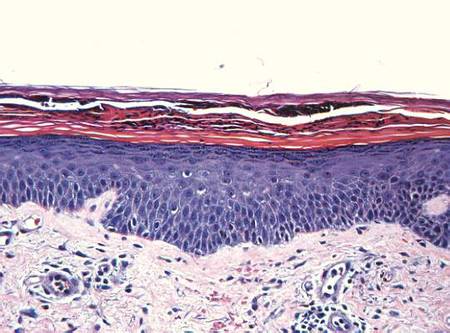
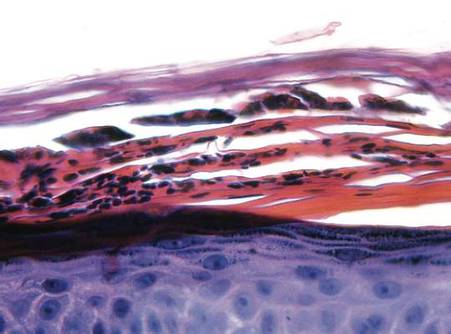
Examination revealed numerous nonfollicular-based pustules on diffuse erythematous patches (Figure 1). There was no mucosal involvement and the skin was negative for the Nikolsky sign. Spongiform intracorneal collections of neutrophils were visible on punch biopsy (Figures 2 and 3). Periodic acid–Schiff stains for fungi were negative.
| 
| |
Figure 2. A punch biopsy showed spongiform intracorneal collections of neutrophils (H&E, original magnification ×200). | Figure 3. A cornified layer of epidermis with neutrophils, as visible on punch biopsy (H&E, original magnification ×630). |
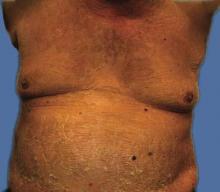
The patient’s primary care physician had initiated a course of oral prednisone 5 mg daily, 3 days before he presented to our outpatient dermatology clinic, but it had little effect on the rash. Upon dermatologic evaluation, we discontinued ranolazine therapy and prescribed the following tapered course of oral prednisone: 60 mg daily for 4 days; 40 mg daily for 3 days; 30 mg daily for 3 days; 20 mg daily for 3 days; 10 mg daily for 3 days; and 5 mg daily for 3 days). Within a week after this regimen was initiated, the rash showed improvement with eventual resolution and desquamation (Figure 4). Subsequently, the patient underwent successful angioplasty and multiple stent placement, which ultimately alleviated his angina.
Comment
Since its original description in 1968,5 AGEP has been misdiagnosed and underreported. Due to its rarity and clinical resemblance to more common pustular eruptions, such as exanthematous pustular psoriasis, the typical characteristics of AGEP were not clearly delineated until Beylot et al3 coined the term AGEP in 1980. Since that time, formalized criteria for the diagnosis and characterization of AGEP have been published.1,2,6-8
Numerous drug therapies have been implicated in the etiology of AGEP, most commonly antimicrobial agents, such as β-lactam antibiotics. Many other drugs, however, also have been identified as potential causative agents,8 including but not limited to antifungal, anticonvulsant, and antihypertensive agents. Other less common etiologies include viral infections,6,9-11 UV radiation, contrast media, heavy metal exposure (eg, to mercury), ingestion of urushiol (eg, in lacquered chicken), and spider bites.2,8,12-16 Nevertheless, more than 90% of AGEP cases are attributed to drug exposure, with 80% of drug-induced cases believed to be caused by antibiotics.1,8
The incidence of AGEP is estimated to be between 1 and 5 cases per million per year, using inclusion criteria from the EuroSCAR study, a multinational, case-controlled, pharmacoepidemiologic study of severe cutaneous adverse reactions.8,16 The condition seems to affect males and females equally.1,4 There are no reports of age or racial predilection.1,6,17 It has been suggested that those with AGEP may have some form of psoriatic background.1 Our patient had no personal history of inflammatory skin disease, although his mother had psoriasis.
The dermatitis presents as the sudden onset of a diffuse exanthematous eruption, which typically produces dozens to hundreds of sterile, nonfollicular, superficial pustules on an erythematous and possibly edematous base. Atypical presentations include target lesions, purpura, and vesicles. The reaction usually begins on the face or intertriginous areas of flexural surfaces and quickly disseminates. Patients may experience burning or pruritus. Acute generalized exanthematous pustulosis may involve mucous membranes but is usually limited to 1 location, most often the oral mucosa.1,8,16,18 Systemic signs and symptoms include fever, lymphadenopathy, pharyngitis, and hepatosplenomegaly. Unlike most drug allergies that demonstrate eosinophilia, AGEP is associated with leukocytosis and neutrophilic predominance. Only 25% of affected patients exhibit eosinophilia.1 Approximately 30% of patients in a retrospective analysis demonstrated abnormal renal function,2 and there have been reports of mildly elevated transaminases.8,19
In the EuroSCAR study, for reasons that were not apparent, symptoms developed within 24 hours of exposure to triggering antibiotics, whereas the median time to rash onset in response to non–anti-infective agents was 11 days.8 This finding is consistent with the delayed onset of symptoms experienced by our patient after initiating ranolazine therapy.
The differential diagnosis of AGEP primarily includes pustular psoriasis, subcorneal pustulosis, pustular folliculitis, DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, bullous impetigo, and occasionally erythema multiforme and toxic epidermal necrolysis, with the latter typically characterized by more mucous membrane involvement.20 Biopsy does not always support a definitive diagnosis; clinical correlation is often necessary. Because of the EuroSCAR study, Sidoroff et al8 devised a clinical validation score based on morphology (presence of pustules and erythema, distribution, and eventual desquamation), histopathology (presence of intraepidermal pustules, spongiosis, and papillary edema), and disease course (duration of symptoms, neutrophilia, fever, acute onset, and time to resolution). A definitive score is 8 to 12 (out of 12), and our patient’s score was 10; the score may have been higher had blood work been performed, but by the time the diagnosis was made the patient’s condition had improved enough to make laboratory workup unnecessary.
Several theories have been proposed to explain the pathophysiology of AGEP. Some hold that the causative agent induces the formation of antigen-antibody complexes, thereby activating the complement system, which in turn produces neutrophil chemotaxis.3,21 A more recent theory suggests that drug exposure causes drug-specific CD4 and CD8 cells to migrate into dermal and epidermal layers of the skin.17 Both T cells and keratinocytes express IL-8, which attracts polymorphonuclear leukocytes, causing them to accumulate in the dermis and then the epidermis. The different clinical presentations of AGEP may be attributed to other cytokines and interleukins that T cells express during this process. In the epidermis, CD8 cells kill keratinocytes, causing focal necrosis and prompting the formation of subcorneal vesicles filled primarily with CD4 cells. CD4 and CD8 cells are then localized to the dermis where neutrophils enter the vesicles, transforming them into sterile pustules.6,16,17
Acute generalized exanthematous pustulosis has been characterized as a type IV delayed hypersensitivity reaction, with affected patients often demonstrating positive patch testing or a history of prior sensitization to the perpetrating agent.18,19,21 Although there have been reports of positive patch testing for certain drugs, the unknown sensitivity and specificity of such testing as well as preparation-dependent variables may limit the diagnostic utility of this approach.21 The additional risk for inducing AGEP by patch testing the suspected drug also is a consideration. Due to our patient’s definitive clinical validation score, we did not perform this test.21
The AGEP eruption is typically self-limited and tends to resolve within 4 to 10 days after cessation of the triggering agent. Postpustular desquamation often occurs upon resolution of the primary lesions. Treatment usually involves discontinuation of the suspected causative agent and the use of antihistamines, antipyretics, topical corticosteroids, and emollients. Although there are reports of AGEP responsiveness to oral and intravenous steroids, such treatment rarely is required.8,16,22 We prescribed a tapered course of oral prednisone due to our patient’s imminent need for angioplasty.
Conclusion
This case of AGEP induced by ranolazine is notable. Given the potential widespread use of this antianginal medication and the severity of this potential adverse reaction, it is important for clinicians to recognize AGEP, discontinue ranolazine if determined to be a causative agent, and then initiate an appropriate alternative antianginal therapy.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Sidoroff A, Halevy S, Bavnick JN, et al. Acute generalized exanthematous pustulosis (AGEP)–a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
3. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases) [in French]. Ann Dermatol Venereol. 1980;107:37-48.
4. Ranexa [package insert]. Foster City, CA: Gilead Sciences, Inc; December 2013.
5. Baker H, Ryan TJ. Generalized pustular psoriasis. a clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80:771-793.
6. Guevara-Gutierrez E, Uribe-Jimenez E, Diaz-Canchola M, et al. Acute generalized exanthematous pustulosis: report of 12 cases and literature review. Int J Dermatol. 2009;48:253-258.
7. Chang SL, Huang YH, Yang CH, et al. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363-365.
8. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) [published online ahead of print September 13, 2007]. Br J Dermatol. 2007;157:989-996.
9. Rouchouse B, Bonnefoy M, Pallot B, et al. Acute generalized exanthematous pustular dermatitis and viral infection. Dermatologica. 1986;173:180-184.
10. Naides SJ, Piette W, Veach LA, et al. Human parvovirus B19-induced vesiculopustular skin eruption. Am J Med. 1988;84:968-972.
11. Feio AB, Apetato M, Costa MM, et al. Acute generalized exanthematous pustulosis due to Coxsackie B4 virus [in Portuguese]. Acta Med Port. 1997;10:487-491.
12. Goh TK, Pang SM, Thirumoorthy T, et al. Acute generalised exanthematous pustulosis and toxic epidermal necrolysis induced by carbamazepine. Singapore Med J. 2008;49:507-510.
13. Ofuji S, Yamamoto O. Acute generalized exanthematous pustulosis associated with a human parvovirus B19 infection. J Dermatol. 2007;34:121-123.
14. Davidovici BB, Pavel D, Cagnano E, et al. Acute generalized exanthematous pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. 2006;55:525-529.
15. Park YM, Park JG, Kang H, et al. Acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Br J Dermatol. 2000;143:230-232.
16. Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis: a case report. Arch Dermatol. 2009;145:683-687.17. Halevy S. Acute generalized exanthematous pustulosis. Curr Opin Allergy Clin Immunol. 2009;9:322-328.
18. Kim HJ, Jung KD, Lee KT, et al. Acute generalized exanthematous pustulosis caused by diltiazem [published online ahead of print February 28, 2011]. Ann Dermatol. 2011;23:108-110.
19. Speck LM, Wilkerson MG, Perri AJ, et al. Acute generalized exanthematous pustulosis caused by terazosin hydrochloride. J Drugs Dermatol. 2008;7:395-397.
20. Sidoroff A. Acute generalized exanthematous pustulosis (AGEP). UpToDate Web site. http://www.uptodate.com /contents/acute-generalized-exanthematous-pustulosis -agep?source=search_result&search=agep&selected Title=1~85. Updated March 18, 2015. Accessed October 6, 2015.
21. Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139:1181-1183.
22. Ibrahimi O, Gunawardane N, Sepehr A, et al. Terbinafine-induced acute generalized exanthematous pustulosis (AGEP) responsive to high dose intravenous corticosteroid. Dermatol Online J. 2009;15:8.
Acute generalized exanthematous pustulosis (AGEP) is a potentially widespread, pustular, cutaneous eruption. In 90% of cases, AGEP results from drug administration.1,2 It manifests as numerous subcorneal, nonfollicular, sterile pustules of rapid onset on an erythematous base,2 often in conjunction with fever, peripheral leukocytosis, and neutrophilia.3 Numerous drug therapies have been implicated in the etiology of AGEP, most commonly the β-lactam antibiotics, such as the penicillin derivatives and cephalosporins.2 Typically, AGEP occurs soon after drug ingestion and resolves spontaneously, shortly after the causative drug is discontinued.
Ranolazine is an antianginal, anti-ischemic medication with an undetermined mechanism of action. Its antianginal and anti-ischemic effects do not depend on reduced heart rate or blood pressure. At therapeutic levels, it inhibits the cardiac late sodium current (INa), reducing the sodium-induced calcium overload in ischemic cardiac myocytes. Severe adverse reactions include angioedema; paresthesia; pancytopenia; and, in animal studies, tumorigenicity.4 Herein we report a case of AGEP associated with the use of ranolazine.
Case Report
An 83-year-old man presented with a generalized rash of approximately 12 days’ duration. The patient reported that the small “pimple-like” bumps initially erupted on the back of the neck but gradually spread to the chest, back, and extremities. The lesions were asymptomatic at the outset and became pruritic over time. For the last several years, the patient had been taking tamsulosin for benign prostatic hypertrophy and rosuvastatin for hyperlipidemia. Twelve days prior to the exanthem, he had started taking ranolazine for symptomatic ischemia until coronary angiography could be performed. He reported having no associated fevers, chills, or malaise and had no personal history of psoriasis, though he had a maternal history of the disorder.
Examination revealed numerous nonfollicular-based pustules on diffuse erythematous patches (Figure 1). There was no mucosal involvement and the skin was negative for the Nikolsky sign. Spongiform intracorneal collections of neutrophils were visible on punch biopsy (Figures 2 and 3). Periodic acid–Schiff stains for fungi were negative.

| 
| |
Figure 2. A punch biopsy showed spongiform intracorneal collections of neutrophils (H&E, original magnification ×200). | Figure 3. A cornified layer of epidermis with neutrophils, as visible on punch biopsy (H&E, original magnification ×630). |
The patient’s primary care physician had initiated a course of oral prednisone 5 mg daily, 3 days before he presented to our outpatient dermatology clinic, but it had little effect on the rash. Upon dermatologic evaluation, we discontinued ranolazine therapy and prescribed the following tapered course of oral prednisone: 60 mg daily for 4 days; 40 mg daily for 3 days; 30 mg daily for 3 days; 20 mg daily for 3 days; 10 mg daily for 3 days; and 5 mg daily for 3 days). Within a week after this regimen was initiated, the rash showed improvement with eventual resolution and desquamation (Figure 4). Subsequently, the patient underwent successful angioplasty and multiple stent placement, which ultimately alleviated his angina.
Comment
Since its original description in 1968,5 AGEP has been misdiagnosed and underreported. Due to its rarity and clinical resemblance to more common pustular eruptions, such as exanthematous pustular psoriasis, the typical characteristics of AGEP were not clearly delineated until Beylot et al3 coined the term AGEP in 1980. Since that time, formalized criteria for the diagnosis and characterization of AGEP have been published.1,2,6-8
Numerous drug therapies have been implicated in the etiology of AGEP, most commonly antimicrobial agents, such as β-lactam antibiotics. Many other drugs, however, also have been identified as potential causative agents,8 including but not limited to antifungal, anticonvulsant, and antihypertensive agents. Other less common etiologies include viral infections,6,9-11 UV radiation, contrast media, heavy metal exposure (eg, to mercury), ingestion of urushiol (eg, in lacquered chicken), and spider bites.2,8,12-16 Nevertheless, more than 90% of AGEP cases are attributed to drug exposure, with 80% of drug-induced cases believed to be caused by antibiotics.1,8
The incidence of AGEP is estimated to be between 1 and 5 cases per million per year, using inclusion criteria from the EuroSCAR study, a multinational, case-controlled, pharmacoepidemiologic study of severe cutaneous adverse reactions.8,16 The condition seems to affect males and females equally.1,4 There are no reports of age or racial predilection.1,6,17 It has been suggested that those with AGEP may have some form of psoriatic background.1 Our patient had no personal history of inflammatory skin disease, although his mother had psoriasis.
The dermatitis presents as the sudden onset of a diffuse exanthematous eruption, which typically produces dozens to hundreds of sterile, nonfollicular, superficial pustules on an erythematous and possibly edematous base. Atypical presentations include target lesions, purpura, and vesicles. The reaction usually begins on the face or intertriginous areas of flexural surfaces and quickly disseminates. Patients may experience burning or pruritus. Acute generalized exanthematous pustulosis may involve mucous membranes but is usually limited to 1 location, most often the oral mucosa.1,8,16,18 Systemic signs and symptoms include fever, lymphadenopathy, pharyngitis, and hepatosplenomegaly. Unlike most drug allergies that demonstrate eosinophilia, AGEP is associated with leukocytosis and neutrophilic predominance. Only 25% of affected patients exhibit eosinophilia.1 Approximately 30% of patients in a retrospective analysis demonstrated abnormal renal function,2 and there have been reports of mildly elevated transaminases.8,19
In the EuroSCAR study, for reasons that were not apparent, symptoms developed within 24 hours of exposure to triggering antibiotics, whereas the median time to rash onset in response to non–anti-infective agents was 11 days.8 This finding is consistent with the delayed onset of symptoms experienced by our patient after initiating ranolazine therapy.
The differential diagnosis of AGEP primarily includes pustular psoriasis, subcorneal pustulosis, pustular folliculitis, DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, bullous impetigo, and occasionally erythema multiforme and toxic epidermal necrolysis, with the latter typically characterized by more mucous membrane involvement.20 Biopsy does not always support a definitive diagnosis; clinical correlation is often necessary. Because of the EuroSCAR study, Sidoroff et al8 devised a clinical validation score based on morphology (presence of pustules and erythema, distribution, and eventual desquamation), histopathology (presence of intraepidermal pustules, spongiosis, and papillary edema), and disease course (duration of symptoms, neutrophilia, fever, acute onset, and time to resolution). A definitive score is 8 to 12 (out of 12), and our patient’s score was 10; the score may have been higher had blood work been performed, but by the time the diagnosis was made the patient’s condition had improved enough to make laboratory workup unnecessary.
Several theories have been proposed to explain the pathophysiology of AGEP. Some hold that the causative agent induces the formation of antigen-antibody complexes, thereby activating the complement system, which in turn produces neutrophil chemotaxis.3,21 A more recent theory suggests that drug exposure causes drug-specific CD4 and CD8 cells to migrate into dermal and epidermal layers of the skin.17 Both T cells and keratinocytes express IL-8, which attracts polymorphonuclear leukocytes, causing them to accumulate in the dermis and then the epidermis. The different clinical presentations of AGEP may be attributed to other cytokines and interleukins that T cells express during this process. In the epidermis, CD8 cells kill keratinocytes, causing focal necrosis and prompting the formation of subcorneal vesicles filled primarily with CD4 cells. CD4 and CD8 cells are then localized to the dermis where neutrophils enter the vesicles, transforming them into sterile pustules.6,16,17
Acute generalized exanthematous pustulosis has been characterized as a type IV delayed hypersensitivity reaction, with affected patients often demonstrating positive patch testing or a history of prior sensitization to the perpetrating agent.18,19,21 Although there have been reports of positive patch testing for certain drugs, the unknown sensitivity and specificity of such testing as well as preparation-dependent variables may limit the diagnostic utility of this approach.21 The additional risk for inducing AGEP by patch testing the suspected drug also is a consideration. Due to our patient’s definitive clinical validation score, we did not perform this test.21
The AGEP eruption is typically self-limited and tends to resolve within 4 to 10 days after cessation of the triggering agent. Postpustular desquamation often occurs upon resolution of the primary lesions. Treatment usually involves discontinuation of the suspected causative agent and the use of antihistamines, antipyretics, topical corticosteroids, and emollients. Although there are reports of AGEP responsiveness to oral and intravenous steroids, such treatment rarely is required.8,16,22 We prescribed a tapered course of oral prednisone due to our patient’s imminent need for angioplasty.
Conclusion
This case of AGEP induced by ranolazine is notable. Given the potential widespread use of this antianginal medication and the severity of this potential adverse reaction, it is important for clinicians to recognize AGEP, discontinue ranolazine if determined to be a causative agent, and then initiate an appropriate alternative antianginal therapy.
Acute generalized exanthematous pustulosis (AGEP) is a potentially widespread, pustular, cutaneous eruption. In 90% of cases, AGEP results from drug administration.1,2 It manifests as numerous subcorneal, nonfollicular, sterile pustules of rapid onset on an erythematous base,2 often in conjunction with fever, peripheral leukocytosis, and neutrophilia.3 Numerous drug therapies have been implicated in the etiology of AGEP, most commonly the β-lactam antibiotics, such as the penicillin derivatives and cephalosporins.2 Typically, AGEP occurs soon after drug ingestion and resolves spontaneously, shortly after the causative drug is discontinued.
Ranolazine is an antianginal, anti-ischemic medication with an undetermined mechanism of action. Its antianginal and anti-ischemic effects do not depend on reduced heart rate or blood pressure. At therapeutic levels, it inhibits the cardiac late sodium current (INa), reducing the sodium-induced calcium overload in ischemic cardiac myocytes. Severe adverse reactions include angioedema; paresthesia; pancytopenia; and, in animal studies, tumorigenicity.4 Herein we report a case of AGEP associated with the use of ranolazine.
Case Report
An 83-year-old man presented with a generalized rash of approximately 12 days’ duration. The patient reported that the small “pimple-like” bumps initially erupted on the back of the neck but gradually spread to the chest, back, and extremities. The lesions were asymptomatic at the outset and became pruritic over time. For the last several years, the patient had been taking tamsulosin for benign prostatic hypertrophy and rosuvastatin for hyperlipidemia. Twelve days prior to the exanthem, he had started taking ranolazine for symptomatic ischemia until coronary angiography could be performed. He reported having no associated fevers, chills, or malaise and had no personal history of psoriasis, though he had a maternal history of the disorder.
Examination revealed numerous nonfollicular-based pustules on diffuse erythematous patches (Figure 1). There was no mucosal involvement and the skin was negative for the Nikolsky sign. Spongiform intracorneal collections of neutrophils were visible on punch biopsy (Figures 2 and 3). Periodic acid–Schiff stains for fungi were negative.

| 
| |
Figure 2. A punch biopsy showed spongiform intracorneal collections of neutrophils (H&E, original magnification ×200). | Figure 3. A cornified layer of epidermis with neutrophils, as visible on punch biopsy (H&E, original magnification ×630). |
The patient’s primary care physician had initiated a course of oral prednisone 5 mg daily, 3 days before he presented to our outpatient dermatology clinic, but it had little effect on the rash. Upon dermatologic evaluation, we discontinued ranolazine therapy and prescribed the following tapered course of oral prednisone: 60 mg daily for 4 days; 40 mg daily for 3 days; 30 mg daily for 3 days; 20 mg daily for 3 days; 10 mg daily for 3 days; and 5 mg daily for 3 days). Within a week after this regimen was initiated, the rash showed improvement with eventual resolution and desquamation (Figure 4). Subsequently, the patient underwent successful angioplasty and multiple stent placement, which ultimately alleviated his angina.
Comment
Since its original description in 1968,5 AGEP has been misdiagnosed and underreported. Due to its rarity and clinical resemblance to more common pustular eruptions, such as exanthematous pustular psoriasis, the typical characteristics of AGEP were not clearly delineated until Beylot et al3 coined the term AGEP in 1980. Since that time, formalized criteria for the diagnosis and characterization of AGEP have been published.1,2,6-8
Numerous drug therapies have been implicated in the etiology of AGEP, most commonly antimicrobial agents, such as β-lactam antibiotics. Many other drugs, however, also have been identified as potential causative agents,8 including but not limited to antifungal, anticonvulsant, and antihypertensive agents. Other less common etiologies include viral infections,6,9-11 UV radiation, contrast media, heavy metal exposure (eg, to mercury), ingestion of urushiol (eg, in lacquered chicken), and spider bites.2,8,12-16 Nevertheless, more than 90% of AGEP cases are attributed to drug exposure, with 80% of drug-induced cases believed to be caused by antibiotics.1,8
The incidence of AGEP is estimated to be between 1 and 5 cases per million per year, using inclusion criteria from the EuroSCAR study, a multinational, case-controlled, pharmacoepidemiologic study of severe cutaneous adverse reactions.8,16 The condition seems to affect males and females equally.1,4 There are no reports of age or racial predilection.1,6,17 It has been suggested that those with AGEP may have some form of psoriatic background.1 Our patient had no personal history of inflammatory skin disease, although his mother had psoriasis.
The dermatitis presents as the sudden onset of a diffuse exanthematous eruption, which typically produces dozens to hundreds of sterile, nonfollicular, superficial pustules on an erythematous and possibly edematous base. Atypical presentations include target lesions, purpura, and vesicles. The reaction usually begins on the face or intertriginous areas of flexural surfaces and quickly disseminates. Patients may experience burning or pruritus. Acute generalized exanthematous pustulosis may involve mucous membranes but is usually limited to 1 location, most often the oral mucosa.1,8,16,18 Systemic signs and symptoms include fever, lymphadenopathy, pharyngitis, and hepatosplenomegaly. Unlike most drug allergies that demonstrate eosinophilia, AGEP is associated with leukocytosis and neutrophilic predominance. Only 25% of affected patients exhibit eosinophilia.1 Approximately 30% of patients in a retrospective analysis demonstrated abnormal renal function,2 and there have been reports of mildly elevated transaminases.8,19
In the EuroSCAR study, for reasons that were not apparent, symptoms developed within 24 hours of exposure to triggering antibiotics, whereas the median time to rash onset in response to non–anti-infective agents was 11 days.8 This finding is consistent with the delayed onset of symptoms experienced by our patient after initiating ranolazine therapy.
The differential diagnosis of AGEP primarily includes pustular psoriasis, subcorneal pustulosis, pustular folliculitis, DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, bullous impetigo, and occasionally erythema multiforme and toxic epidermal necrolysis, with the latter typically characterized by more mucous membrane involvement.20 Biopsy does not always support a definitive diagnosis; clinical correlation is often necessary. Because of the EuroSCAR study, Sidoroff et al8 devised a clinical validation score based on morphology (presence of pustules and erythema, distribution, and eventual desquamation), histopathology (presence of intraepidermal pustules, spongiosis, and papillary edema), and disease course (duration of symptoms, neutrophilia, fever, acute onset, and time to resolution). A definitive score is 8 to 12 (out of 12), and our patient’s score was 10; the score may have been higher had blood work been performed, but by the time the diagnosis was made the patient’s condition had improved enough to make laboratory workup unnecessary.
Several theories have been proposed to explain the pathophysiology of AGEP. Some hold that the causative agent induces the formation of antigen-antibody complexes, thereby activating the complement system, which in turn produces neutrophil chemotaxis.3,21 A more recent theory suggests that drug exposure causes drug-specific CD4 and CD8 cells to migrate into dermal and epidermal layers of the skin.17 Both T cells and keratinocytes express IL-8, which attracts polymorphonuclear leukocytes, causing them to accumulate in the dermis and then the epidermis. The different clinical presentations of AGEP may be attributed to other cytokines and interleukins that T cells express during this process. In the epidermis, CD8 cells kill keratinocytes, causing focal necrosis and prompting the formation of subcorneal vesicles filled primarily with CD4 cells. CD4 and CD8 cells are then localized to the dermis where neutrophils enter the vesicles, transforming them into sterile pustules.6,16,17
Acute generalized exanthematous pustulosis has been characterized as a type IV delayed hypersensitivity reaction, with affected patients often demonstrating positive patch testing or a history of prior sensitization to the perpetrating agent.18,19,21 Although there have been reports of positive patch testing for certain drugs, the unknown sensitivity and specificity of such testing as well as preparation-dependent variables may limit the diagnostic utility of this approach.21 The additional risk for inducing AGEP by patch testing the suspected drug also is a consideration. Due to our patient’s definitive clinical validation score, we did not perform this test.21
The AGEP eruption is typically self-limited and tends to resolve within 4 to 10 days after cessation of the triggering agent. Postpustular desquamation often occurs upon resolution of the primary lesions. Treatment usually involves discontinuation of the suspected causative agent and the use of antihistamines, antipyretics, topical corticosteroids, and emollients. Although there are reports of AGEP responsiveness to oral and intravenous steroids, such treatment rarely is required.8,16,22 We prescribed a tapered course of oral prednisone due to our patient’s imminent need for angioplasty.
Conclusion
This case of AGEP induced by ranolazine is notable. Given the potential widespread use of this antianginal medication and the severity of this potential adverse reaction, it is important for clinicians to recognize AGEP, discontinue ranolazine if determined to be a causative agent, and then initiate an appropriate alternative antianginal therapy.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Sidoroff A, Halevy S, Bavnick JN, et al. Acute generalized exanthematous pustulosis (AGEP)–a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
3. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases) [in French]. Ann Dermatol Venereol. 1980;107:37-48.
4. Ranexa [package insert]. Foster City, CA: Gilead Sciences, Inc; December 2013.
5. Baker H, Ryan TJ. Generalized pustular psoriasis. a clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80:771-793.
6. Guevara-Gutierrez E, Uribe-Jimenez E, Diaz-Canchola M, et al. Acute generalized exanthematous pustulosis: report of 12 cases and literature review. Int J Dermatol. 2009;48:253-258.
7. Chang SL, Huang YH, Yang CH, et al. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363-365.
8. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) [published online ahead of print September 13, 2007]. Br J Dermatol. 2007;157:989-996.
9. Rouchouse B, Bonnefoy M, Pallot B, et al. Acute generalized exanthematous pustular dermatitis and viral infection. Dermatologica. 1986;173:180-184.
10. Naides SJ, Piette W, Veach LA, et al. Human parvovirus B19-induced vesiculopustular skin eruption. Am J Med. 1988;84:968-972.
11. Feio AB, Apetato M, Costa MM, et al. Acute generalized exanthematous pustulosis due to Coxsackie B4 virus [in Portuguese]. Acta Med Port. 1997;10:487-491.
12. Goh TK, Pang SM, Thirumoorthy T, et al. Acute generalised exanthematous pustulosis and toxic epidermal necrolysis induced by carbamazepine. Singapore Med J. 2008;49:507-510.
13. Ofuji S, Yamamoto O. Acute generalized exanthematous pustulosis associated with a human parvovirus B19 infection. J Dermatol. 2007;34:121-123.
14. Davidovici BB, Pavel D, Cagnano E, et al. Acute generalized exanthematous pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. 2006;55:525-529.
15. Park YM, Park JG, Kang H, et al. Acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Br J Dermatol. 2000;143:230-232.
16. Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis: a case report. Arch Dermatol. 2009;145:683-687.17. Halevy S. Acute generalized exanthematous pustulosis. Curr Opin Allergy Clin Immunol. 2009;9:322-328.
18. Kim HJ, Jung KD, Lee KT, et al. Acute generalized exanthematous pustulosis caused by diltiazem [published online ahead of print February 28, 2011]. Ann Dermatol. 2011;23:108-110.
19. Speck LM, Wilkerson MG, Perri AJ, et al. Acute generalized exanthematous pustulosis caused by terazosin hydrochloride. J Drugs Dermatol. 2008;7:395-397.
20. Sidoroff A. Acute generalized exanthematous pustulosis (AGEP). UpToDate Web site. http://www.uptodate.com /contents/acute-generalized-exanthematous-pustulosis -agep?source=search_result&search=agep&selected Title=1~85. Updated March 18, 2015. Accessed October 6, 2015.
21. Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139:1181-1183.
22. Ibrahimi O, Gunawardane N, Sepehr A, et al. Terbinafine-induced acute generalized exanthematous pustulosis (AGEP) responsive to high dose intravenous corticosteroid. Dermatol Online J. 2009;15:8.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Sidoroff A, Halevy S, Bavnick JN, et al. Acute generalized exanthematous pustulosis (AGEP)–a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
3. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases) [in French]. Ann Dermatol Venereol. 1980;107:37-48.
4. Ranexa [package insert]. Foster City, CA: Gilead Sciences, Inc; December 2013.
5. Baker H, Ryan TJ. Generalized pustular psoriasis. a clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80:771-793.
6. Guevara-Gutierrez E, Uribe-Jimenez E, Diaz-Canchola M, et al. Acute generalized exanthematous pustulosis: report of 12 cases and literature review. Int J Dermatol. 2009;48:253-258.
7. Chang SL, Huang YH, Yang CH, et al. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363-365.
8. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) [published online ahead of print September 13, 2007]. Br J Dermatol. 2007;157:989-996.
9. Rouchouse B, Bonnefoy M, Pallot B, et al. Acute generalized exanthematous pustular dermatitis and viral infection. Dermatologica. 1986;173:180-184.
10. Naides SJ, Piette W, Veach LA, et al. Human parvovirus B19-induced vesiculopustular skin eruption. Am J Med. 1988;84:968-972.
11. Feio AB, Apetato M, Costa MM, et al. Acute generalized exanthematous pustulosis due to Coxsackie B4 virus [in Portuguese]. Acta Med Port. 1997;10:487-491.
12. Goh TK, Pang SM, Thirumoorthy T, et al. Acute generalised exanthematous pustulosis and toxic epidermal necrolysis induced by carbamazepine. Singapore Med J. 2008;49:507-510.
13. Ofuji S, Yamamoto O. Acute generalized exanthematous pustulosis associated with a human parvovirus B19 infection. J Dermatol. 2007;34:121-123.
14. Davidovici BB, Pavel D, Cagnano E, et al. Acute generalized exanthematous pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. 2006;55:525-529.
15. Park YM, Park JG, Kang H, et al. Acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Br J Dermatol. 2000;143:230-232.
16. Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis: a case report. Arch Dermatol. 2009;145:683-687.17. Halevy S. Acute generalized exanthematous pustulosis. Curr Opin Allergy Clin Immunol. 2009;9:322-328.
18. Kim HJ, Jung KD, Lee KT, et al. Acute generalized exanthematous pustulosis caused by diltiazem [published online ahead of print February 28, 2011]. Ann Dermatol. 2011;23:108-110.
19. Speck LM, Wilkerson MG, Perri AJ, et al. Acute generalized exanthematous pustulosis caused by terazosin hydrochloride. J Drugs Dermatol. 2008;7:395-397.
20. Sidoroff A. Acute generalized exanthematous pustulosis (AGEP). UpToDate Web site. http://www.uptodate.com /contents/acute-generalized-exanthematous-pustulosis -agep?source=search_result&search=agep&selected Title=1~85. Updated March 18, 2015. Accessed October 6, 2015.
21. Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139:1181-1183.
22. Ibrahimi O, Gunawardane N, Sepehr A, et al. Terbinafine-induced acute generalized exanthematous pustulosis (AGEP) responsive to high dose intravenous corticosteroid. Dermatol Online J. 2009;15:8.
Practice Points
- Encountering an acute pustular reaction pattern should trigger the clinician to rule out acute generalized exanthematous pustulosis (AGEP).
- Ranolazine, a new antianginal therapy, has been associated with AGEP.
- Upon confirmation of AGEP, the patient’s recent medication history should be reviewed so the potential causative agent can be identified and withdrawn.