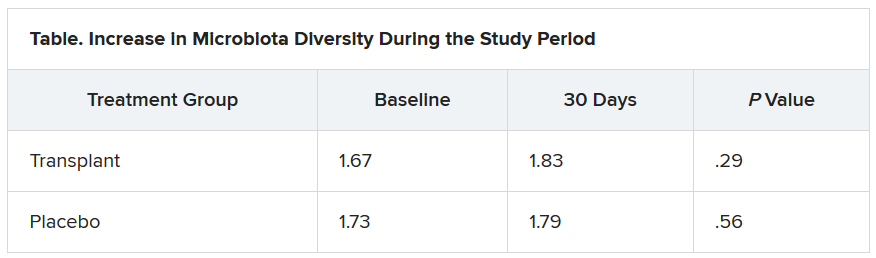User login
Tools emerging to predict liver failure in cirrhosis
Systemic inflammation and portal hypertension are key predictors of acute-on-chronic liver failure (ACLF) in the 3 months after a hospital stay for acute decompensated cirrhosis and also of death after 12 months, a preliminary analysis of data from the PREDICT study shows.
“Before this, we never had any patient signatures to identify ACLF,” said Jonel Trebicka, MD, PhD, from the JW Goethe University Hospital in Frankfurt, Germany.
Now, Dr. Trebicka’s team has “characterized the phenotypes in pre-ACLF that will progress within 3 months,” he said in an interview. “Those with high levels of inflammatory proteins, white blood cell count, are more likely to develop ACLF.”
ACLF is a highly complex disorder that can lead liver, cardiovascular, renal, cerebral, pulmonary, intestinal, adrenal, and immune systems to fail, Dr. Trebicka explained when he discussed the analysis – published online in the Journal of Hepatology – during the virtual International Liver Congress (ILC) 2020.
The chance of survival after the onset of ACLF is low – the 28-day survival rate is 30% – and “the only treatment we have is liver transplant,” he said.
For their prospective observational study, Dr. Trebicka and his colleagues assessed 1071 participants from 48 European hospitals in 14 countries who were admitted for an episode of acute decompensation, defined as the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, infection, or a combination thereof.
The researchers identified three distinct clinical courses for a patient hospitalized with acute decompensated cirrhosis that will help clinicians predict the development of ACLF.
At study enrollment, more than half of the patients at highest risk for ACLF had pre-ACLF and high-grade systemic inflammation. The patients at intermediate risk had unstable decompensated cirrhosis with low-grade systemic inflammation and complications related to severe portal hypertension. And those at lowest risk for ACLF had stable decompensated cirrhosis and no severe systemic inflammation or portal hypertension complications, and did not develop ACLF or another episode of acute decompensation in the subsequent 3 months.
“There have been hints of possible phenotypes before – for stable and unstable ACLF – but we never had anything specific to diagnose,” Trebicka reported.
“We found that there are two main mechanisms in the development of ACLF that are most important,” he said. The first is systemic inflammation with high levels of proteins, which “leads to organ failure. This is the most striking acute mechanism.”
The second is the development of portal hypertension. “This is slower, but also very important, causing increased pressure in the portal vein, and leading to bleeding if the pressure is too great,” he said.
More tools emerging to help predict ACLF
The Albumin-functionality-test (AFT), which uses serum albumin levels to evaluate liver and kidney function, might also be useful in the prediction of ACLF and 12-month survival, according to a separate study an Italian group presented at the virtual ILC.
“Our main results are that parameters from albumin predict the development of ACLF in acute decompensated patients with the same diagnostic performance as the CLIF-AD score,” said Katja Waterstradt, PhD, from the University of Bologna in Italy.
And when the two tests are combined, diagnostic performance is increased, she added.
Dr. Trebicka has disclosed no relevant financial relationships. Dr. Waterstrand is a researcher for MedInnovation GmbH.
This article first appeared on Medscape.com.
Systemic inflammation and portal hypertension are key predictors of acute-on-chronic liver failure (ACLF) in the 3 months after a hospital stay for acute decompensated cirrhosis and also of death after 12 months, a preliminary analysis of data from the PREDICT study shows.
“Before this, we never had any patient signatures to identify ACLF,” said Jonel Trebicka, MD, PhD, from the JW Goethe University Hospital in Frankfurt, Germany.
Now, Dr. Trebicka’s team has “characterized the phenotypes in pre-ACLF that will progress within 3 months,” he said in an interview. “Those with high levels of inflammatory proteins, white blood cell count, are more likely to develop ACLF.”
ACLF is a highly complex disorder that can lead liver, cardiovascular, renal, cerebral, pulmonary, intestinal, adrenal, and immune systems to fail, Dr. Trebicka explained when he discussed the analysis – published online in the Journal of Hepatology – during the virtual International Liver Congress (ILC) 2020.
The chance of survival after the onset of ACLF is low – the 28-day survival rate is 30% – and “the only treatment we have is liver transplant,” he said.
For their prospective observational study, Dr. Trebicka and his colleagues assessed 1071 participants from 48 European hospitals in 14 countries who were admitted for an episode of acute decompensation, defined as the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, infection, or a combination thereof.
The researchers identified three distinct clinical courses for a patient hospitalized with acute decompensated cirrhosis that will help clinicians predict the development of ACLF.
At study enrollment, more than half of the patients at highest risk for ACLF had pre-ACLF and high-grade systemic inflammation. The patients at intermediate risk had unstable decompensated cirrhosis with low-grade systemic inflammation and complications related to severe portal hypertension. And those at lowest risk for ACLF had stable decompensated cirrhosis and no severe systemic inflammation or portal hypertension complications, and did not develop ACLF or another episode of acute decompensation in the subsequent 3 months.
“There have been hints of possible phenotypes before – for stable and unstable ACLF – but we never had anything specific to diagnose,” Trebicka reported.
“We found that there are two main mechanisms in the development of ACLF that are most important,” he said. The first is systemic inflammation with high levels of proteins, which “leads to organ failure. This is the most striking acute mechanism.”
The second is the development of portal hypertension. “This is slower, but also very important, causing increased pressure in the portal vein, and leading to bleeding if the pressure is too great,” he said.
More tools emerging to help predict ACLF
The Albumin-functionality-test (AFT), which uses serum albumin levels to evaluate liver and kidney function, might also be useful in the prediction of ACLF and 12-month survival, according to a separate study an Italian group presented at the virtual ILC.
“Our main results are that parameters from albumin predict the development of ACLF in acute decompensated patients with the same diagnostic performance as the CLIF-AD score,” said Katja Waterstradt, PhD, from the University of Bologna in Italy.
And when the two tests are combined, diagnostic performance is increased, she added.
Dr. Trebicka has disclosed no relevant financial relationships. Dr. Waterstrand is a researcher for MedInnovation GmbH.
This article first appeared on Medscape.com.
Systemic inflammation and portal hypertension are key predictors of acute-on-chronic liver failure (ACLF) in the 3 months after a hospital stay for acute decompensated cirrhosis and also of death after 12 months, a preliminary analysis of data from the PREDICT study shows.
“Before this, we never had any patient signatures to identify ACLF,” said Jonel Trebicka, MD, PhD, from the JW Goethe University Hospital in Frankfurt, Germany.
Now, Dr. Trebicka’s team has “characterized the phenotypes in pre-ACLF that will progress within 3 months,” he said in an interview. “Those with high levels of inflammatory proteins, white blood cell count, are more likely to develop ACLF.”
ACLF is a highly complex disorder that can lead liver, cardiovascular, renal, cerebral, pulmonary, intestinal, adrenal, and immune systems to fail, Dr. Trebicka explained when he discussed the analysis – published online in the Journal of Hepatology – during the virtual International Liver Congress (ILC) 2020.
The chance of survival after the onset of ACLF is low – the 28-day survival rate is 30% – and “the only treatment we have is liver transplant,” he said.
For their prospective observational study, Dr. Trebicka and his colleagues assessed 1071 participants from 48 European hospitals in 14 countries who were admitted for an episode of acute decompensation, defined as the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, infection, or a combination thereof.
The researchers identified three distinct clinical courses for a patient hospitalized with acute decompensated cirrhosis that will help clinicians predict the development of ACLF.
At study enrollment, more than half of the patients at highest risk for ACLF had pre-ACLF and high-grade systemic inflammation. The patients at intermediate risk had unstable decompensated cirrhosis with low-grade systemic inflammation and complications related to severe portal hypertension. And those at lowest risk for ACLF had stable decompensated cirrhosis and no severe systemic inflammation or portal hypertension complications, and did not develop ACLF or another episode of acute decompensation in the subsequent 3 months.
“There have been hints of possible phenotypes before – for stable and unstable ACLF – but we never had anything specific to diagnose,” Trebicka reported.
“We found that there are two main mechanisms in the development of ACLF that are most important,” he said. The first is systemic inflammation with high levels of proteins, which “leads to organ failure. This is the most striking acute mechanism.”
The second is the development of portal hypertension. “This is slower, but also very important, causing increased pressure in the portal vein, and leading to bleeding if the pressure is too great,” he said.
More tools emerging to help predict ACLF
The Albumin-functionality-test (AFT), which uses serum albumin levels to evaluate liver and kidney function, might also be useful in the prediction of ACLF and 12-month survival, according to a separate study an Italian group presented at the virtual ILC.
“Our main results are that parameters from albumin predict the development of ACLF in acute decompensated patients with the same diagnostic performance as the CLIF-AD score,” said Katja Waterstradt, PhD, from the University of Bologna in Italy.
And when the two tests are combined, diagnostic performance is increased, she added.
Dr. Trebicka has disclosed no relevant financial relationships. Dr. Waterstrand is a researcher for MedInnovation GmbH.
This article first appeared on Medscape.com.
Many advanced countries missing targets for HCV elimination
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
Help your patients better understand the risks and treatment for hepatitis C by sharing AGA GI Patient Center education at http://ow.ly/xV2S30r8L29.
A version of this article originally appeared on Medscape.com.
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
Help your patients better understand the risks and treatment for hepatitis C by sharing AGA GI Patient Center education at http://ow.ly/xV2S30r8L29.
A version of this article originally appeared on Medscape.com.
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
Help your patients better understand the risks and treatment for hepatitis C by sharing AGA GI Patient Center education at http://ow.ly/xV2S30r8L29.
A version of this article originally appeared on Medscape.com.
Many advanced countries missing targets for HCV elimination
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Repeat FIB-4 blood tests help predict cirrhosis
Repeat Fibrosis-4 (FIB-4) scores can be used to identify people at greatest risk for cirrhosis of the liver, new research shows.
“Done repeatedly, this test can improve prediction capacity to identify who will develop cirrhosis of the liver later in life,” said lead researcher Hannes Hagström, MD, from the Karolinska University Hospital in Stockholm.
A FIB-4 score that rises from one test to the next indicates that a person is at increased risk for severe liver disease, whereas a score that drops indicates a decreased risk, he told Medscape Medical News. The study results — published online July 1 in the Journal of Hepatology, was presented at the Digital International Liver Congress 2020.
The noninvasive, widely available, cheap FIB-4 test — which is calculated on the basis of age, transaminase level, and platelet count — is commonly used to identify the risk for advanced fibrosis in liver disease, but it has not been used to predict future risk.
To evaluate risk for cirrhosis, Hagström and his colleagues looked at 812,073 blood tests performed from 1985 to 1996 on people enrolled in the Swedish Apolipoprotein Mortality Risk (AMORIS) study.
They excluded people younger than 35 years and older than 79 years and anyone with a diagnosis of any liver disease at baseline.
The 40,729 people who had two FIB-4 measurements taken less than 5 years apart were included in the analysis. Test results were categorized into three risk groups: low (<1.30), intermediate (1.30 - 2.67), and high (>2.67).
After a median of 16.2 years, 11,929 people in the study cohort had died and 581 had a severe liver disease event.
Severe liver disease events were more common in people who had both tests categorized as high risk than in people who had both tests categorized as low risk (13.2% vs 1.0%; aHR, 17.04; 95% CI, 11.67 - 24.88).
The researchers found that a one-unit increase between the two test results was continuously predictive of a severe liver disease event (aHR, 1.81; 95% CI, 1.67 - 1.96).
One test not enough
The absolute risk for severe liver disease in the general population is 2%, but the FIB-4 score is elevated in about one-third of people in the general population.
“A lot of people who have increased levels of this biomarker will never develop cirrhosis,” Hagström told Medscape Medical News.
Although two FIB-4 scores might not identify everyone who will get cirrhosis, comparing scores provides insight into who is at greatest risk, he explained.
This information can be useful, particularly for primary care doctors. If you know that someone is at higher risk, “you can send that patient for a FibroScan, which is a much more sensitive measurement,” but also much more expensive. “Now we can better know who to send,” he said.
However, “the main problem is that these tests are not widely known” or used enough by primary care doctors, Hagström said.
A lack of knowledge about the utility of this test is a problem, agreed Jérôme Boursier, MD, PhD, from Angers University in France.
“The younger doctors are using these tests more often,” he told Medscape Medical News, but “the older doctors are not aware they exist.”
This study supports repeating the tests. “One test offers quite poor prediction,” Boursier said. But “when you have a higher score on a second one, this can help the conversation with the patient.”
Hagström and Boursier have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Repeat Fibrosis-4 (FIB-4) scores can be used to identify people at greatest risk for cirrhosis of the liver, new research shows.
“Done repeatedly, this test can improve prediction capacity to identify who will develop cirrhosis of the liver later in life,” said lead researcher Hannes Hagström, MD, from the Karolinska University Hospital in Stockholm.
A FIB-4 score that rises from one test to the next indicates that a person is at increased risk for severe liver disease, whereas a score that drops indicates a decreased risk, he told Medscape Medical News. The study results — published online July 1 in the Journal of Hepatology, was presented at the Digital International Liver Congress 2020.
The noninvasive, widely available, cheap FIB-4 test — which is calculated on the basis of age, transaminase level, and platelet count — is commonly used to identify the risk for advanced fibrosis in liver disease, but it has not been used to predict future risk.
To evaluate risk for cirrhosis, Hagström and his colleagues looked at 812,073 blood tests performed from 1985 to 1996 on people enrolled in the Swedish Apolipoprotein Mortality Risk (AMORIS) study.
They excluded people younger than 35 years and older than 79 years and anyone with a diagnosis of any liver disease at baseline.
The 40,729 people who had two FIB-4 measurements taken less than 5 years apart were included in the analysis. Test results were categorized into three risk groups: low (<1.30), intermediate (1.30 - 2.67), and high (>2.67).
After a median of 16.2 years, 11,929 people in the study cohort had died and 581 had a severe liver disease event.
Severe liver disease events were more common in people who had both tests categorized as high risk than in people who had both tests categorized as low risk (13.2% vs 1.0%; aHR, 17.04; 95% CI, 11.67 - 24.88).
The researchers found that a one-unit increase between the two test results was continuously predictive of a severe liver disease event (aHR, 1.81; 95% CI, 1.67 - 1.96).
One test not enough
The absolute risk for severe liver disease in the general population is 2%, but the FIB-4 score is elevated in about one-third of people in the general population.
“A lot of people who have increased levels of this biomarker will never develop cirrhosis,” Hagström told Medscape Medical News.
Although two FIB-4 scores might not identify everyone who will get cirrhosis, comparing scores provides insight into who is at greatest risk, he explained.
This information can be useful, particularly for primary care doctors. If you know that someone is at higher risk, “you can send that patient for a FibroScan, which is a much more sensitive measurement,” but also much more expensive. “Now we can better know who to send,” he said.
However, “the main problem is that these tests are not widely known” or used enough by primary care doctors, Hagström said.
A lack of knowledge about the utility of this test is a problem, agreed Jérôme Boursier, MD, PhD, from Angers University in France.
“The younger doctors are using these tests more often,” he told Medscape Medical News, but “the older doctors are not aware they exist.”
This study supports repeating the tests. “One test offers quite poor prediction,” Boursier said. But “when you have a higher score on a second one, this can help the conversation with the patient.”
Hagström and Boursier have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Repeat Fibrosis-4 (FIB-4) scores can be used to identify people at greatest risk for cirrhosis of the liver, new research shows.
“Done repeatedly, this test can improve prediction capacity to identify who will develop cirrhosis of the liver later in life,” said lead researcher Hannes Hagström, MD, from the Karolinska University Hospital in Stockholm.
A FIB-4 score that rises from one test to the next indicates that a person is at increased risk for severe liver disease, whereas a score that drops indicates a decreased risk, he told Medscape Medical News. The study results — published online July 1 in the Journal of Hepatology, was presented at the Digital International Liver Congress 2020.
The noninvasive, widely available, cheap FIB-4 test — which is calculated on the basis of age, transaminase level, and platelet count — is commonly used to identify the risk for advanced fibrosis in liver disease, but it has not been used to predict future risk.
To evaluate risk for cirrhosis, Hagström and his colleagues looked at 812,073 blood tests performed from 1985 to 1996 on people enrolled in the Swedish Apolipoprotein Mortality Risk (AMORIS) study.
They excluded people younger than 35 years and older than 79 years and anyone with a diagnosis of any liver disease at baseline.
The 40,729 people who had two FIB-4 measurements taken less than 5 years apart were included in the analysis. Test results were categorized into three risk groups: low (<1.30), intermediate (1.30 - 2.67), and high (>2.67).
After a median of 16.2 years, 11,929 people in the study cohort had died and 581 had a severe liver disease event.
Severe liver disease events were more common in people who had both tests categorized as high risk than in people who had both tests categorized as low risk (13.2% vs 1.0%; aHR, 17.04; 95% CI, 11.67 - 24.88).
The researchers found that a one-unit increase between the two test results was continuously predictive of a severe liver disease event (aHR, 1.81; 95% CI, 1.67 - 1.96).
One test not enough
The absolute risk for severe liver disease in the general population is 2%, but the FIB-4 score is elevated in about one-third of people in the general population.
“A lot of people who have increased levels of this biomarker will never develop cirrhosis,” Hagström told Medscape Medical News.
Although two FIB-4 scores might not identify everyone who will get cirrhosis, comparing scores provides insight into who is at greatest risk, he explained.
This information can be useful, particularly for primary care doctors. If you know that someone is at higher risk, “you can send that patient for a FibroScan, which is a much more sensitive measurement,” but also much more expensive. “Now we can better know who to send,” he said.
However, “the main problem is that these tests are not widely known” or used enough by primary care doctors, Hagström said.
A lack of knowledge about the utility of this test is a problem, agreed Jérôme Boursier, MD, PhD, from Angers University in France.
“The younger doctors are using these tests more often,” he told Medscape Medical News, but “the older doctors are not aware they exist.”
This study supports repeating the tests. “One test offers quite poor prediction,” Boursier said. But “when you have a higher score on a second one, this can help the conversation with the patient.”
Hagström and Boursier have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Fecal transplant shows promise in reducing alcohol craving
Fecal microbiota transplantation results in a short-term reduction in alcohol craving in patients with alcohol-induced cirrhosis who can’t stop drinking, results from a new study show.
And that reduction could lead to a better psychosocial quality of life for patients with cirrhosis and alcohol use disorder, said investigator Jasmohan Bajaj, MD, from Virginia Commonwealth University, Richmond.
“This is the most common addiction disorder worldwide, but we have nothing to treat these patients with,” he said.
Cirrhosis is associated with an altered gut-brain axis. It leads to organ damage in several parts of the body, including the brain, gut, pancreas, and liver. This makes changing the gut microbes “an attractive target,” Dr. Bajaj said at the Digital International Liver Congress 2020.
For their phase 1, double-blind study, he and his colleagues assessed 20 men from a Virginia veteran’s hospital with untreatable alcohol use disorder who were not eligible for liver transplantation.
All had failed behavioral or pharmacologic therapy and were unwilling to try again. “That’s what made them good candidates to try something new,” Dr. Bajaj said during a press briefing.
Mean age in the study cohort was 65 years, mean Model for End-Stage Liver disease score was 8.9, and demographic characteristics were similar between the 10 men randomly assigned to fecal transplantation and the 10 assigned to placebo. One man in each group dropped out of the study.
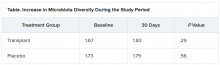
The investigators evaluated cravings, microbiota, and quality of life during the 30-day study period.
At day 15, significantly more men in the transplant group than in the placebo group experienced a reduction in alcohol cravings (90% vs. 30%).
At 30 days, levels of creatinine, serum interleukin-6, and lipopolysaccharide-binding protein were lower in the transplant group than in the placebo group. In addition, levels of butyrate and isobutyrate increased, as did cognition and quality of life scores.
There was also a decrease in urinary ethyl glucuronide in the transplant group, which “is the objective criteria for alcohol intake,” Dr. Bajaj reported, noting that there was no change in ethyl glucuronide in the placebo group.
The increase in microbiota diversity was significant in the transplant group but not in the placebo group. Alistipes, Odoribacter, and Roseburia were more abundant in the transplant group than in the placebo group.
During the 30-day study period, two men in the placebo group required medical attention, one for hyponatremia and the other for atrial fibrillation. However, no adverse events were seen in any men in the transplant group. “This was the No. 1 result,” Dr. Bajaj said.
Liver disease and the microbiome
“Understanding of interactions between the human and microbiome genome [metagenome] in health and disease has represented one of the major areas of progress in the last few years,” said Luca Valenti, MD, from the University of Milan, who is a member of the scientific committee of the European Association the Study of the Liver, which organized the congress.
“These studies lay the groundwork for the exploitation of this new knowledge for the treatment of liver disease,” he said.
“We are [now] diagnosing liver disease and the stages of liver disease based on microbiome changes,” said Jonel Trebicka, MD, PhD, from University Hospital Frankfurt (Germany), who chaired a session at the congress on the role of the microbiome in liver disease.
“This and other studies have shown us that the microbiome itself may influence liver disease,” he added.
Dr. Bajaj is considered one of the world’s experts on cirrhosis and the microbiome, Dr. Trebicka explained. Last year, Dr. Bajaj and his team demonstrated that fecal microbiota transplantation can reduce the incidence of recurrent hepatic encephalopathy, as reported by Medscape Medical News.
The current study also “shows clearly that the microbiome plays a role in craving. FMT reduces the desire for alcohol,” said Dr. Trebicka.
“The way to the brain is through the gut,” Dr. Bajaj said.
Dr. Bajaj, Dr. Trebicka, and Dr. Valenti disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Fecal microbiota transplantation results in a short-term reduction in alcohol craving in patients with alcohol-induced cirrhosis who can’t stop drinking, results from a new study show.
And that reduction could lead to a better psychosocial quality of life for patients with cirrhosis and alcohol use disorder, said investigator Jasmohan Bajaj, MD, from Virginia Commonwealth University, Richmond.
“This is the most common addiction disorder worldwide, but we have nothing to treat these patients with,” he said.
Cirrhosis is associated with an altered gut-brain axis. It leads to organ damage in several parts of the body, including the brain, gut, pancreas, and liver. This makes changing the gut microbes “an attractive target,” Dr. Bajaj said at the Digital International Liver Congress 2020.
For their phase 1, double-blind study, he and his colleagues assessed 20 men from a Virginia veteran’s hospital with untreatable alcohol use disorder who were not eligible for liver transplantation.
All had failed behavioral or pharmacologic therapy and were unwilling to try again. “That’s what made them good candidates to try something new,” Dr. Bajaj said during a press briefing.
Mean age in the study cohort was 65 years, mean Model for End-Stage Liver disease score was 8.9, and demographic characteristics were similar between the 10 men randomly assigned to fecal transplantation and the 10 assigned to placebo. One man in each group dropped out of the study.
The investigators evaluated cravings, microbiota, and quality of life during the 30-day study period.
At day 15, significantly more men in the transplant group than in the placebo group experienced a reduction in alcohol cravings (90% vs. 30%).
At 30 days, levels of creatinine, serum interleukin-6, and lipopolysaccharide-binding protein were lower in the transplant group than in the placebo group. In addition, levels of butyrate and isobutyrate increased, as did cognition and quality of life scores.
There was also a decrease in urinary ethyl glucuronide in the transplant group, which “is the objective criteria for alcohol intake,” Dr. Bajaj reported, noting that there was no change in ethyl glucuronide in the placebo group.
The increase in microbiota diversity was significant in the transplant group but not in the placebo group. Alistipes, Odoribacter, and Roseburia were more abundant in the transplant group than in the placebo group.
During the 30-day study period, two men in the placebo group required medical attention, one for hyponatremia and the other for atrial fibrillation. However, no adverse events were seen in any men in the transplant group. “This was the No. 1 result,” Dr. Bajaj said.
Liver disease and the microbiome
“Understanding of interactions between the human and microbiome genome [metagenome] in health and disease has represented one of the major areas of progress in the last few years,” said Luca Valenti, MD, from the University of Milan, who is a member of the scientific committee of the European Association the Study of the Liver, which organized the congress.
“These studies lay the groundwork for the exploitation of this new knowledge for the treatment of liver disease,” he said.
“We are [now] diagnosing liver disease and the stages of liver disease based on microbiome changes,” said Jonel Trebicka, MD, PhD, from University Hospital Frankfurt (Germany), who chaired a session at the congress on the role of the microbiome in liver disease.
“This and other studies have shown us that the microbiome itself may influence liver disease,” he added.
Dr. Bajaj is considered one of the world’s experts on cirrhosis and the microbiome, Dr. Trebicka explained. Last year, Dr. Bajaj and his team demonstrated that fecal microbiota transplantation can reduce the incidence of recurrent hepatic encephalopathy, as reported by Medscape Medical News.
The current study also “shows clearly that the microbiome plays a role in craving. FMT reduces the desire for alcohol,” said Dr. Trebicka.
“The way to the brain is through the gut,” Dr. Bajaj said.
Dr. Bajaj, Dr. Trebicka, and Dr. Valenti disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Fecal microbiota transplantation results in a short-term reduction in alcohol craving in patients with alcohol-induced cirrhosis who can’t stop drinking, results from a new study show.
And that reduction could lead to a better psychosocial quality of life for patients with cirrhosis and alcohol use disorder, said investigator Jasmohan Bajaj, MD, from Virginia Commonwealth University, Richmond.
“This is the most common addiction disorder worldwide, but we have nothing to treat these patients with,” he said.
Cirrhosis is associated with an altered gut-brain axis. It leads to organ damage in several parts of the body, including the brain, gut, pancreas, and liver. This makes changing the gut microbes “an attractive target,” Dr. Bajaj said at the Digital International Liver Congress 2020.
For their phase 1, double-blind study, he and his colleagues assessed 20 men from a Virginia veteran’s hospital with untreatable alcohol use disorder who were not eligible for liver transplantation.
All had failed behavioral or pharmacologic therapy and were unwilling to try again. “That’s what made them good candidates to try something new,” Dr. Bajaj said during a press briefing.
Mean age in the study cohort was 65 years, mean Model for End-Stage Liver disease score was 8.9, and demographic characteristics were similar between the 10 men randomly assigned to fecal transplantation and the 10 assigned to placebo. One man in each group dropped out of the study.
The investigators evaluated cravings, microbiota, and quality of life during the 30-day study period.
At day 15, significantly more men in the transplant group than in the placebo group experienced a reduction in alcohol cravings (90% vs. 30%).
At 30 days, levels of creatinine, serum interleukin-6, and lipopolysaccharide-binding protein were lower in the transplant group than in the placebo group. In addition, levels of butyrate and isobutyrate increased, as did cognition and quality of life scores.
There was also a decrease in urinary ethyl glucuronide in the transplant group, which “is the objective criteria for alcohol intake,” Dr. Bajaj reported, noting that there was no change in ethyl glucuronide in the placebo group.
The increase in microbiota diversity was significant in the transplant group but not in the placebo group. Alistipes, Odoribacter, and Roseburia were more abundant in the transplant group than in the placebo group.
During the 30-day study period, two men in the placebo group required medical attention, one for hyponatremia and the other for atrial fibrillation. However, no adverse events were seen in any men in the transplant group. “This was the No. 1 result,” Dr. Bajaj said.
Liver disease and the microbiome
“Understanding of interactions between the human and microbiome genome [metagenome] in health and disease has represented one of the major areas of progress in the last few years,” said Luca Valenti, MD, from the University of Milan, who is a member of the scientific committee of the European Association the Study of the Liver, which organized the congress.
“These studies lay the groundwork for the exploitation of this new knowledge for the treatment of liver disease,” he said.
“We are [now] diagnosing liver disease and the stages of liver disease based on microbiome changes,” said Jonel Trebicka, MD, PhD, from University Hospital Frankfurt (Germany), who chaired a session at the congress on the role of the microbiome in liver disease.
“This and other studies have shown us that the microbiome itself may influence liver disease,” he added.
Dr. Bajaj is considered one of the world’s experts on cirrhosis and the microbiome, Dr. Trebicka explained. Last year, Dr. Bajaj and his team demonstrated that fecal microbiota transplantation can reduce the incidence of recurrent hepatic encephalopathy, as reported by Medscape Medical News.
The current study also “shows clearly that the microbiome plays a role in craving. FMT reduces the desire for alcohol,” said Dr. Trebicka.
“The way to the brain is through the gut,” Dr. Bajaj said.
Dr. Bajaj, Dr. Trebicka, and Dr. Valenti disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Pulmonary rehab reduces COPD readmissions
Pulmonary rehabilitation reduces the likelihood that patients with chronic obstructive pulmonary disease (COPD) will be readmitted to the hospital in the year after discharge by 33%, new research shows, but few patients participate in those programs.
In fact, in a retrospective cohort of 197,376 patients from 4446 hospitals, only 1.5% of patients initiated pulmonary rehabilitation in the 90 days after hospital discharge.
“This is a striking finding,” said Mihaela Stefan, PhD, from the University of Massachusetts Medical School–Baystate in Springfield. “Our study demonstrates that we need to increase access to rehabilitation to reduce the risk of readmissions.”
Not enough patients are initiating rehabilitation, but the onus is not only on them; the system is failing them. “We wanted to understand how much pulmonary rehabilitation lowers the readmission rate,” Stefan told Medscape Medical News.
So she and her colleagues examined the records of patients who were hospitalized for COPD in 2014 to see whether they had begun rehabilitation in the 90 days after discharge and whether they were readmitted to the hospital in the subsequent 12 months.
Patients who were unlikely to initiate pulmonary rehabilitation — such as those with dementia or metastatic cancer and those discharged to hospice care or a nursing home — were excluded from the analysis, Stefan said during her presentation at the study results at the virtual American Thoracic Society (ATS) 2020 International Conference.
The risk analysis was complex because many patients died before the year was out, and “a patient who dies has no risk of being readmitted,” she explained. Selection bias was also a factor because patients who do pulmonary rehab tend to be in better shape.
The researchers used propensity score matching and Anderson–Gill models of cumulative rehospitalizations or death at 1 year with time-varying exposure to pulmonary rehabilitation to account for clustering of individual events and adjust for covariates. “It was a complicated risk analysis,” she said.
In the year after discharge, 130,660 patients (66%) were readmitted to the hospital. The rate of rehospitalization was lower for those who initiated rehabilitation than for those who did not (59% vs 66%), as was the mean number of readmissions per patient (1.4 vs 1.8).
Rehabilitation was associated with a lower risk for readmission or death (hazard ratio, 0.67; 95% CI, 0.66 - 0.69).
“We know the referral rates are low and that pulmonary rehabilitation is effective in clinical trials,” said Stefan, and now “we see that pulmonary rehabilitation is effective when you look at patients in real life.”
From a provider perspective, “we need to make sure that hospitals get more money for pulmonary rehabilitation. Cardiac rehabilitation is paid for,” she explained. "But pulmonary rehab is not a lucrative business. I don›t know why the CMS pays more for cardiac."
A rehabilitation program generally consists of 36 sessions, held two or three times a week, and many patients can’t afford that on their own, she noted. Transportation is another huge issue.
A recent study in which semi-structured interviews were conducted with 15 COPD patients showed that the main barriers to enrollment in a pulmonary rehabilitation program are lack of awareness, family obligations, transportation, and lack of motivation, said Stefan, who was involved in that research.
Telehealth rehabilitation programs might become more available in the near future, given the COVID pandemic. But “currently, Medicare doesn’t pay for telerehab,” she said. Virtual sessions might attract more patients, but lack of computer access and training could present another barrier for some.
PAH rehab
Uptake for pulmonary rehabilitation is as low for patients with pulmonary arterial hypertension (PAH) as it is for those with COPD, according to another study presented at the virtual ATS meeting.
An examination of the electronic health records of 111,356 veterans who experienced incident PAH from 2010 to 2016 showed that only 1,737 (1.6%) followed through on pulmonary rehabilitation.
“Exercise therapy is safe and effective at improving outcomes,” lead author Thomas Cascino, MD, from the University of Michigan in Ann Arbor, said in an ATS press release. “Recognizing that it is being underutilized is a necessary first step in working toward increasing patient access to rehab.
His group is currently working on a trial for home-based rehabilitation “using wearable technology as a means to expand access for people unable to come to center-based rehab for a variety of reasons,” he explained.
“The goal of all our treatments is to help people feel better and live longer,” Cascino added.
Stefan and Cascino have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Pulmonary rehabilitation reduces the likelihood that patients with chronic obstructive pulmonary disease (COPD) will be readmitted to the hospital in the year after discharge by 33%, new research shows, but few patients participate in those programs.
In fact, in a retrospective cohort of 197,376 patients from 4446 hospitals, only 1.5% of patients initiated pulmonary rehabilitation in the 90 days after hospital discharge.
“This is a striking finding,” said Mihaela Stefan, PhD, from the University of Massachusetts Medical School–Baystate in Springfield. “Our study demonstrates that we need to increase access to rehabilitation to reduce the risk of readmissions.”
Not enough patients are initiating rehabilitation, but the onus is not only on them; the system is failing them. “We wanted to understand how much pulmonary rehabilitation lowers the readmission rate,” Stefan told Medscape Medical News.
So she and her colleagues examined the records of patients who were hospitalized for COPD in 2014 to see whether they had begun rehabilitation in the 90 days after discharge and whether they were readmitted to the hospital in the subsequent 12 months.
Patients who were unlikely to initiate pulmonary rehabilitation — such as those with dementia or metastatic cancer and those discharged to hospice care or a nursing home — were excluded from the analysis, Stefan said during her presentation at the study results at the virtual American Thoracic Society (ATS) 2020 International Conference.
The risk analysis was complex because many patients died before the year was out, and “a patient who dies has no risk of being readmitted,” she explained. Selection bias was also a factor because patients who do pulmonary rehab tend to be in better shape.
The researchers used propensity score matching and Anderson–Gill models of cumulative rehospitalizations or death at 1 year with time-varying exposure to pulmonary rehabilitation to account for clustering of individual events and adjust for covariates. “It was a complicated risk analysis,” she said.
In the year after discharge, 130,660 patients (66%) were readmitted to the hospital. The rate of rehospitalization was lower for those who initiated rehabilitation than for those who did not (59% vs 66%), as was the mean number of readmissions per patient (1.4 vs 1.8).
Rehabilitation was associated with a lower risk for readmission or death (hazard ratio, 0.67; 95% CI, 0.66 - 0.69).
“We know the referral rates are low and that pulmonary rehabilitation is effective in clinical trials,” said Stefan, and now “we see that pulmonary rehabilitation is effective when you look at patients in real life.”
From a provider perspective, “we need to make sure that hospitals get more money for pulmonary rehabilitation. Cardiac rehabilitation is paid for,” she explained. "But pulmonary rehab is not a lucrative business. I don›t know why the CMS pays more for cardiac."
A rehabilitation program generally consists of 36 sessions, held two or three times a week, and many patients can’t afford that on their own, she noted. Transportation is another huge issue.
A recent study in which semi-structured interviews were conducted with 15 COPD patients showed that the main barriers to enrollment in a pulmonary rehabilitation program are lack of awareness, family obligations, transportation, and lack of motivation, said Stefan, who was involved in that research.
Telehealth rehabilitation programs might become more available in the near future, given the COVID pandemic. But “currently, Medicare doesn’t pay for telerehab,” she said. Virtual sessions might attract more patients, but lack of computer access and training could present another barrier for some.
PAH rehab
Uptake for pulmonary rehabilitation is as low for patients with pulmonary arterial hypertension (PAH) as it is for those with COPD, according to another study presented at the virtual ATS meeting.
An examination of the electronic health records of 111,356 veterans who experienced incident PAH from 2010 to 2016 showed that only 1,737 (1.6%) followed through on pulmonary rehabilitation.
“Exercise therapy is safe and effective at improving outcomes,” lead author Thomas Cascino, MD, from the University of Michigan in Ann Arbor, said in an ATS press release. “Recognizing that it is being underutilized is a necessary first step in working toward increasing patient access to rehab.
His group is currently working on a trial for home-based rehabilitation “using wearable technology as a means to expand access for people unable to come to center-based rehab for a variety of reasons,” he explained.
“The goal of all our treatments is to help people feel better and live longer,” Cascino added.
Stefan and Cascino have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Pulmonary rehabilitation reduces the likelihood that patients with chronic obstructive pulmonary disease (COPD) will be readmitted to the hospital in the year after discharge by 33%, new research shows, but few patients participate in those programs.
In fact, in a retrospective cohort of 197,376 patients from 4446 hospitals, only 1.5% of patients initiated pulmonary rehabilitation in the 90 days after hospital discharge.
“This is a striking finding,” said Mihaela Stefan, PhD, from the University of Massachusetts Medical School–Baystate in Springfield. “Our study demonstrates that we need to increase access to rehabilitation to reduce the risk of readmissions.”
Not enough patients are initiating rehabilitation, but the onus is not only on them; the system is failing them. “We wanted to understand how much pulmonary rehabilitation lowers the readmission rate,” Stefan told Medscape Medical News.
So she and her colleagues examined the records of patients who were hospitalized for COPD in 2014 to see whether they had begun rehabilitation in the 90 days after discharge and whether they were readmitted to the hospital in the subsequent 12 months.
Patients who were unlikely to initiate pulmonary rehabilitation — such as those with dementia or metastatic cancer and those discharged to hospice care or a nursing home — were excluded from the analysis, Stefan said during her presentation at the study results at the virtual American Thoracic Society (ATS) 2020 International Conference.
The risk analysis was complex because many patients died before the year was out, and “a patient who dies has no risk of being readmitted,” she explained. Selection bias was also a factor because patients who do pulmonary rehab tend to be in better shape.
The researchers used propensity score matching and Anderson–Gill models of cumulative rehospitalizations or death at 1 year with time-varying exposure to pulmonary rehabilitation to account for clustering of individual events and adjust for covariates. “It was a complicated risk analysis,” she said.
In the year after discharge, 130,660 patients (66%) were readmitted to the hospital. The rate of rehospitalization was lower for those who initiated rehabilitation than for those who did not (59% vs 66%), as was the mean number of readmissions per patient (1.4 vs 1.8).
Rehabilitation was associated with a lower risk for readmission or death (hazard ratio, 0.67; 95% CI, 0.66 - 0.69).
“We know the referral rates are low and that pulmonary rehabilitation is effective in clinical trials,” said Stefan, and now “we see that pulmonary rehabilitation is effective when you look at patients in real life.”
From a provider perspective, “we need to make sure that hospitals get more money for pulmonary rehabilitation. Cardiac rehabilitation is paid for,” she explained. "But pulmonary rehab is not a lucrative business. I don›t know why the CMS pays more for cardiac."
A rehabilitation program generally consists of 36 sessions, held two or three times a week, and many patients can’t afford that on their own, she noted. Transportation is another huge issue.
A recent study in which semi-structured interviews were conducted with 15 COPD patients showed that the main barriers to enrollment in a pulmonary rehabilitation program are lack of awareness, family obligations, transportation, and lack of motivation, said Stefan, who was involved in that research.
Telehealth rehabilitation programs might become more available in the near future, given the COVID pandemic. But “currently, Medicare doesn’t pay for telerehab,” she said. Virtual sessions might attract more patients, but lack of computer access and training could present another barrier for some.
PAH rehab
Uptake for pulmonary rehabilitation is as low for patients with pulmonary arterial hypertension (PAH) as it is for those with COPD, according to another study presented at the virtual ATS meeting.
An examination of the electronic health records of 111,356 veterans who experienced incident PAH from 2010 to 2016 showed that only 1,737 (1.6%) followed through on pulmonary rehabilitation.
“Exercise therapy is safe and effective at improving outcomes,” lead author Thomas Cascino, MD, from the University of Michigan in Ann Arbor, said in an ATS press release. “Recognizing that it is being underutilized is a necessary first step in working toward increasing patient access to rehab.
His group is currently working on a trial for home-based rehabilitation “using wearable technology as a means to expand access for people unable to come to center-based rehab for a variety of reasons,” he explained.
“The goal of all our treatments is to help people feel better and live longer,” Cascino added.
Stefan and Cascino have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Beyond PSA: New prostate cancer screening options
Two noninvasive tests — an assessment of spermine levels in urine and a blood test that combines free and total PSA and the (-2) pro-PSA isoform (p2PSA) — are much safer than historically risky biopsy and what is now considered to have been unnecessary surgery.
“We’ve ‘cured’ a lot of men,” Franklin Gaylis, MD, from the University of California, San Diego, told Medscape Medical News. “Even some who didn’t need to be cured.” Now, we are working to solve this dilemma, he said. “It’s time we determine who do you screen, [who do you] not screen, and how aggressively?”
Urine Spermine Test More Accurate Than PSA
Data from a highly predictive test that assesses spermine levels in urine were presented by Peter Ka-Fung Chiu, MD, from the University of Hong Kong, at the virtual annual congress of the European Association of Urology. Normal spermine levels are inversely associated with both prostate cancer (PCa) and high-grade prostate cancer (HGPCa).
To investigate the predictive value of spermine for any PCa or HGPCa (Gleason 7 or above), the researchers recruited 556 men from two centers and collected 30 mL of urine prior to prostate biopsy.
They analyzed data from 390 men and used decision-curve analyses for PCa and for HGPCa. The multivariate spermine score — which takes into account age, prostate volume, PSA level, and spermine level — provided net clinical benefit over PSA alone and over spermine score alone.
“At 90% sensitivity, this risk score actually had a negative predictive value of 96.7% and avoided about 50% of unnecessary biopsies,” Chiu explained. “This test predicts prostate cancer and high-grade prostate cancer well, without the need for prior prostate massage, offering improved predictive performance.”
PHI Reduces Need for MRI Screening
Another test, the PHI prostate cancer biomarker, is as predictive as multiparametric (mp)MRI, both with and without PSA scoring.
PHI scores from 554 men from five centers added to either PSA density or mpMRI improved the prediction of risk for ≥GG2 cancers to more than 0.81 and for ≥CPG3 cancers to more than 0.85, according to data from the multicenter PRIM (PHI to Refine MRI) study group recently published in BMC Medicine and presented at EAU.
With a PHI cut-off of 30, mpMRI referrals could be cut by 25%, and unnecessary biopsies could be cut by 40%, the PRIM group reports. PHI misses 8% of ≥GG2 cancers, whereas mpMRI misses 9%.
The PHI strategy reduces “mpMRI and biopsies without compromising detection of significant prostate cancers,” and also reduces costs, Nicholas Boxall, MB ChB, from Cambridge University Hospitals NHS Foundation Trust in the United Kingdom, explained during his presentation
“Instead of screening everyone, we’re risk-adapting who needs to be screened, identifying the right population and defaulting to MRI as an alternative to invasive biopsy, and doing secondary tests to look at biomarkers,” said Gerald Andriole, MD, from the Washington University School of Medicine in St. Louis, Missouri.
“We don’t have to auto-toggle to aggressive treatment,” he told Medscape Medical News. “We’re getting better than we were 10 years ago, but we need slightly better tests, and we also need better biopsies; urologists must be more careful.”
Chiu and Boxall report no relevant financial relationships. Gaylis is a scientific advisor for Stratify Genomics. Andriole is on the advisory board of Stratify Genomics.
This article first appeared on Medscape.com.
Two noninvasive tests — an assessment of spermine levels in urine and a blood test that combines free and total PSA and the (-2) pro-PSA isoform (p2PSA) — are much safer than historically risky biopsy and what is now considered to have been unnecessary surgery.
“We’ve ‘cured’ a lot of men,” Franklin Gaylis, MD, from the University of California, San Diego, told Medscape Medical News. “Even some who didn’t need to be cured.” Now, we are working to solve this dilemma, he said. “It’s time we determine who do you screen, [who do you] not screen, and how aggressively?”
Urine Spermine Test More Accurate Than PSA
Data from a highly predictive test that assesses spermine levels in urine were presented by Peter Ka-Fung Chiu, MD, from the University of Hong Kong, at the virtual annual congress of the European Association of Urology. Normal spermine levels are inversely associated with both prostate cancer (PCa) and high-grade prostate cancer (HGPCa).
To investigate the predictive value of spermine for any PCa or HGPCa (Gleason 7 or above), the researchers recruited 556 men from two centers and collected 30 mL of urine prior to prostate biopsy.
They analyzed data from 390 men and used decision-curve analyses for PCa and for HGPCa. The multivariate spermine score — which takes into account age, prostate volume, PSA level, and spermine level — provided net clinical benefit over PSA alone and over spermine score alone.
“At 90% sensitivity, this risk score actually had a negative predictive value of 96.7% and avoided about 50% of unnecessary biopsies,” Chiu explained. “This test predicts prostate cancer and high-grade prostate cancer well, without the need for prior prostate massage, offering improved predictive performance.”
PHI Reduces Need for MRI Screening
Another test, the PHI prostate cancer biomarker, is as predictive as multiparametric (mp)MRI, both with and without PSA scoring.
PHI scores from 554 men from five centers added to either PSA density or mpMRI improved the prediction of risk for ≥GG2 cancers to more than 0.81 and for ≥CPG3 cancers to more than 0.85, according to data from the multicenter PRIM (PHI to Refine MRI) study group recently published in BMC Medicine and presented at EAU.
With a PHI cut-off of 30, mpMRI referrals could be cut by 25%, and unnecessary biopsies could be cut by 40%, the PRIM group reports. PHI misses 8% of ≥GG2 cancers, whereas mpMRI misses 9%.
The PHI strategy reduces “mpMRI and biopsies without compromising detection of significant prostate cancers,” and also reduces costs, Nicholas Boxall, MB ChB, from Cambridge University Hospitals NHS Foundation Trust in the United Kingdom, explained during his presentation
“Instead of screening everyone, we’re risk-adapting who needs to be screened, identifying the right population and defaulting to MRI as an alternative to invasive biopsy, and doing secondary tests to look at biomarkers,” said Gerald Andriole, MD, from the Washington University School of Medicine in St. Louis, Missouri.
“We don’t have to auto-toggle to aggressive treatment,” he told Medscape Medical News. “We’re getting better than we were 10 years ago, but we need slightly better tests, and we also need better biopsies; urologists must be more careful.”
Chiu and Boxall report no relevant financial relationships. Gaylis is a scientific advisor for Stratify Genomics. Andriole is on the advisory board of Stratify Genomics.
This article first appeared on Medscape.com.
Two noninvasive tests — an assessment of spermine levels in urine and a blood test that combines free and total PSA and the (-2) pro-PSA isoform (p2PSA) — are much safer than historically risky biopsy and what is now considered to have been unnecessary surgery.
“We’ve ‘cured’ a lot of men,” Franklin Gaylis, MD, from the University of California, San Diego, told Medscape Medical News. “Even some who didn’t need to be cured.” Now, we are working to solve this dilemma, he said. “It’s time we determine who do you screen, [who do you] not screen, and how aggressively?”
Urine Spermine Test More Accurate Than PSA
Data from a highly predictive test that assesses spermine levels in urine were presented by Peter Ka-Fung Chiu, MD, from the University of Hong Kong, at the virtual annual congress of the European Association of Urology. Normal spermine levels are inversely associated with both prostate cancer (PCa) and high-grade prostate cancer (HGPCa).
To investigate the predictive value of spermine for any PCa or HGPCa (Gleason 7 or above), the researchers recruited 556 men from two centers and collected 30 mL of urine prior to prostate biopsy.
They analyzed data from 390 men and used decision-curve analyses for PCa and for HGPCa. The multivariate spermine score — which takes into account age, prostate volume, PSA level, and spermine level — provided net clinical benefit over PSA alone and over spermine score alone.
“At 90% sensitivity, this risk score actually had a negative predictive value of 96.7% and avoided about 50% of unnecessary biopsies,” Chiu explained. “This test predicts prostate cancer and high-grade prostate cancer well, without the need for prior prostate massage, offering improved predictive performance.”
PHI Reduces Need for MRI Screening
Another test, the PHI prostate cancer biomarker, is as predictive as multiparametric (mp)MRI, both with and without PSA scoring.
PHI scores from 554 men from five centers added to either PSA density or mpMRI improved the prediction of risk for ≥GG2 cancers to more than 0.81 and for ≥CPG3 cancers to more than 0.85, according to data from the multicenter PRIM (PHI to Refine MRI) study group recently published in BMC Medicine and presented at EAU.
With a PHI cut-off of 30, mpMRI referrals could be cut by 25%, and unnecessary biopsies could be cut by 40%, the PRIM group reports. PHI misses 8% of ≥GG2 cancers, whereas mpMRI misses 9%.
The PHI strategy reduces “mpMRI and biopsies without compromising detection of significant prostate cancers,” and also reduces costs, Nicholas Boxall, MB ChB, from Cambridge University Hospitals NHS Foundation Trust in the United Kingdom, explained during his presentation
“Instead of screening everyone, we’re risk-adapting who needs to be screened, identifying the right population and defaulting to MRI as an alternative to invasive biopsy, and doing secondary tests to look at biomarkers,” said Gerald Andriole, MD, from the Washington University School of Medicine in St. Louis, Missouri.
“We don’t have to auto-toggle to aggressive treatment,” he told Medscape Medical News. “We’re getting better than we were 10 years ago, but we need slightly better tests, and we also need better biopsies; urologists must be more careful.”
Chiu and Boxall report no relevant financial relationships. Gaylis is a scientific advisor for Stratify Genomics. Andriole is on the advisory board of Stratify Genomics.
This article first appeared on Medscape.com.
MRI reliably identifies significant prostate cancer
Prostate cancers that are missed on multiparametric (mp) MRI are small and “not life-threatening,” according to an analysis of data from the Prostate MR Imaging Study (PROMIS).
“Our work suggests that MRI scans of the prostate appear to deliver crucial information about a man’s risk of dying from prostate cancer, even before he has a biopsy,” said Joseph Norris, BM BS, from University College London.
“This may mean that we can finally move prostate cancer to a position in which we can use imaging as the primary tool to direct further investigations, treatment, and prediction of risk,” he told Medscape Medical News.
This is “a position that all other solid organ cancers have reached,” said Norris, who will present the findings at the upcoming virtual European Association of Urology 2020 Congress.
All 576 PROMIS participants underwent an mpMRI scan, a transrectal ultrasonography (TRUS)–guided biopsy, and a template prostate mapping (TPM) biopsy taken at 5-mm intervals across the entire prostate.
PROMIS researchers previously showed that mpMRI had a 93% sensitivity for clinically significant cancer, whereas TRUS biopsy had only a 48% sensitivity, as reported by Medscape Medical News. And they concluded that the use of mpMRI as a first-line diagnostic tool could prevent 27% of all biopsies, which can have serious adverse effects, such as pain, urinary problems, infection, bleeding, and erectile dysfunction.
However, in their study looking at the accuracy of mpMRI and TRUS biopsy, the researchers did not investigate the severity of the 7% of cancers that mpMRI missed. “What if those missed cancers are, in fact, aggressive? That’s what we set out to examine,” Norris explained.
So he and his colleagues conducted a post ad hoc analysis of the PROMIS participants in whom clinically significant cancer had been detected with TPM biopsy to see which of those cancers had been detected with mpMRI. The findings were published online in European Urology.
Cancers met the strict definition of clinically significant if they had a Gleason score of at least 4+3 for a tumor of any length, or a maximum cancer core length (MCCL) greater than 6 mm for a cancer of any grade. They met the less-strict definition if they had a Gleason score of at least 3+4 for a tumor of any length, or a MCCL greater than 4 mm for a cancer of any grade.
In PROMIS, TPM biopsy detected 230 cancers that met the strict definition of clinically significant and 331 that met the less-strict definition.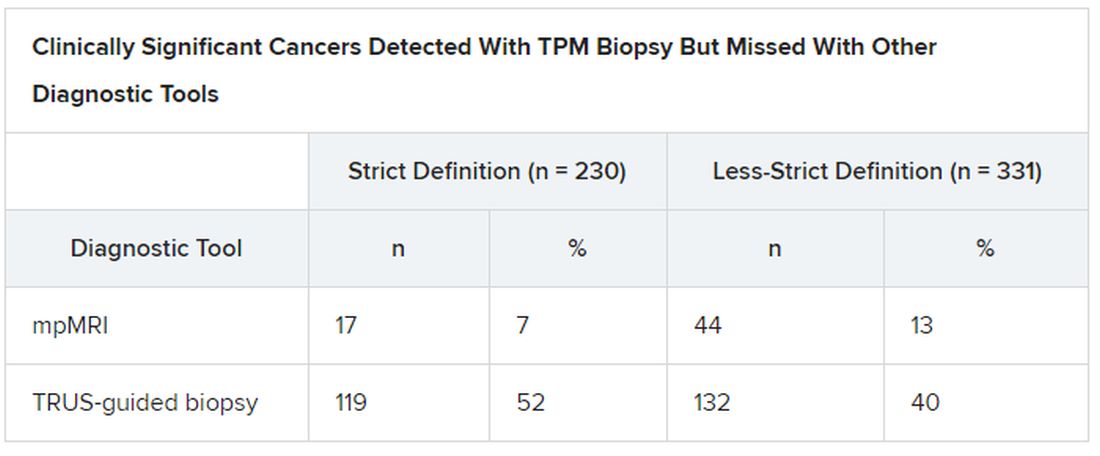
Overall Gleason scores were significantly lower for the 17 strict-definition cancers not detected with 1.5 T mpMRI than for those detected with mpMRI (P = .0007), as were maximum Gleason scores (P < .0001).
Median MCCL was 3 mm shorter for all 17 tumors missed with mpMRI than for those detected with mpMRI (5 vs 8 mm; P < .0001).
mpMRI detected all tumors identified on TPM biopsy that had an overall Gleason score greater than 3+4 (Gleason grades 3 to 5) or a maximum Gleason score greater than 4+3 (Gleason grades 4 and 5).
“This finding is important, given that in PROMIS, no men with an overall Gleason score of 4+3 had cancer missed by MRI, indicating that actually MRI may be able to identify all truly significant cancers,” said Norris.
Adding PSA Density Threshold
To further assess cancers missed on mpMRI, the researchers looked at prostate-specific antigen (PSA) density, calculated as total PSA level (ng/mL) divided by prostate volume (mL).
“We found that if we applied a threshold PSA density to men with normal-looking MRI scans, we could reduce the proportion of missed significant cancer to just 5%. This is exciting; it means we can make MRI an even more effective test for prostate cancer in a very simple way,” Norris reported.
“These data show that no highly aggressive prostate cancers were missed by MRI, either at the level of the whole prostate or at the individual needle level,” said Norris. This should lead to positive outcomes in the long term.
And since the PROMIS data were gathered, MRI technology has improved, he said. The “MRI scanners in PROMIS were 1.5 Tesla,” whereas today’s machines are 3.0 T, which could increase the detection of significant prostate cancer.
In fact, “our analysis here potentially overestimates the amount of undetected disease,” he noted.
Prostate cancer that is not clinically significant is often monitored with active surveillance, so “invisible” cancers missed on mpMRI could actually be looked at in a positive light, he explained.
Variation in technique, interpretation
But the quality of care when it comes to the diagnosis of prostate cancer is not equal everywhere, said Gerald Andriole, MD, from the Washington School of Medicine in St. Louis, Missouri.
“When you get an MRI in a center that doesn’t do a lot of prostate cancer testing, you may not have the best software and you may have a radiologist who is not that experienced,” he told Medscape Medical News. Specialized cancer centers of excellence tend to do a great job finding prostate cancer, “but other centers have high significant-miss rates or high overcall rates.”
“The elephant in the room remains the considerable variation in technique and interobserver interpretation of prostate mpMRI,” write Steven Monda, MD, and Marc Dall’Era, MD, both from UC Davis Health in Sacramento, California, in an editorial that accompanies the new PROMIS analysis.
“These problems must be addressed and remedied in each institution before relying on results from PROMIS to drive changes in clinical practice,” they add.
Norris, Andriole, Monda, and Dall’Era have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Prostate cancers that are missed on multiparametric (mp) MRI are small and “not life-threatening,” according to an analysis of data from the Prostate MR Imaging Study (PROMIS).
“Our work suggests that MRI scans of the prostate appear to deliver crucial information about a man’s risk of dying from prostate cancer, even before he has a biopsy,” said Joseph Norris, BM BS, from University College London.
“This may mean that we can finally move prostate cancer to a position in which we can use imaging as the primary tool to direct further investigations, treatment, and prediction of risk,” he told Medscape Medical News.
This is “a position that all other solid organ cancers have reached,” said Norris, who will present the findings at the upcoming virtual European Association of Urology 2020 Congress.
All 576 PROMIS participants underwent an mpMRI scan, a transrectal ultrasonography (TRUS)–guided biopsy, and a template prostate mapping (TPM) biopsy taken at 5-mm intervals across the entire prostate.
PROMIS researchers previously showed that mpMRI had a 93% sensitivity for clinically significant cancer, whereas TRUS biopsy had only a 48% sensitivity, as reported by Medscape Medical News. And they concluded that the use of mpMRI as a first-line diagnostic tool could prevent 27% of all biopsies, which can have serious adverse effects, such as pain, urinary problems, infection, bleeding, and erectile dysfunction.
However, in their study looking at the accuracy of mpMRI and TRUS biopsy, the researchers did not investigate the severity of the 7% of cancers that mpMRI missed. “What if those missed cancers are, in fact, aggressive? That’s what we set out to examine,” Norris explained.
So he and his colleagues conducted a post ad hoc analysis of the PROMIS participants in whom clinically significant cancer had been detected with TPM biopsy to see which of those cancers had been detected with mpMRI. The findings were published online in European Urology.
Cancers met the strict definition of clinically significant if they had a Gleason score of at least 4+3 for a tumor of any length, or a maximum cancer core length (MCCL) greater than 6 mm for a cancer of any grade. They met the less-strict definition if they had a Gleason score of at least 3+4 for a tumor of any length, or a MCCL greater than 4 mm for a cancer of any grade.
In PROMIS, TPM biopsy detected 230 cancers that met the strict definition of clinically significant and 331 that met the less-strict definition.
Overall Gleason scores were significantly lower for the 17 strict-definition cancers not detected with 1.5 T mpMRI than for those detected with mpMRI (P = .0007), as were maximum Gleason scores (P < .0001).
Median MCCL was 3 mm shorter for all 17 tumors missed with mpMRI than for those detected with mpMRI (5 vs 8 mm; P < .0001).
mpMRI detected all tumors identified on TPM biopsy that had an overall Gleason score greater than 3+4 (Gleason grades 3 to 5) or a maximum Gleason score greater than 4+3 (Gleason grades 4 and 5).
“This finding is important, given that in PROMIS, no men with an overall Gleason score of 4+3 had cancer missed by MRI, indicating that actually MRI may be able to identify all truly significant cancers,” said Norris.
Adding PSA Density Threshold
To further assess cancers missed on mpMRI, the researchers looked at prostate-specific antigen (PSA) density, calculated as total PSA level (ng/mL) divided by prostate volume (mL).
“We found that if we applied a threshold PSA density to men with normal-looking MRI scans, we could reduce the proportion of missed significant cancer to just 5%. This is exciting; it means we can make MRI an even more effective test for prostate cancer in a very simple way,” Norris reported.
“These data show that no highly aggressive prostate cancers were missed by MRI, either at the level of the whole prostate or at the individual needle level,” said Norris. This should lead to positive outcomes in the long term.
And since the PROMIS data were gathered, MRI technology has improved, he said. The “MRI scanners in PROMIS were 1.5 Tesla,” whereas today’s machines are 3.0 T, which could increase the detection of significant prostate cancer.
In fact, “our analysis here potentially overestimates the amount of undetected disease,” he noted.
Prostate cancer that is not clinically significant is often monitored with active surveillance, so “invisible” cancers missed on mpMRI could actually be looked at in a positive light, he explained.
Variation in technique, interpretation
But the quality of care when it comes to the diagnosis of prostate cancer is not equal everywhere, said Gerald Andriole, MD, from the Washington School of Medicine in St. Louis, Missouri.
“When you get an MRI in a center that doesn’t do a lot of prostate cancer testing, you may not have the best software and you may have a radiologist who is not that experienced,” he told Medscape Medical News. Specialized cancer centers of excellence tend to do a great job finding prostate cancer, “but other centers have high significant-miss rates or high overcall rates.”
“The elephant in the room remains the considerable variation in technique and interobserver interpretation of prostate mpMRI,” write Steven Monda, MD, and Marc Dall’Era, MD, both from UC Davis Health in Sacramento, California, in an editorial that accompanies the new PROMIS analysis.
“These problems must be addressed and remedied in each institution before relying on results from PROMIS to drive changes in clinical practice,” they add.
Norris, Andriole, Monda, and Dall’Era have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Prostate cancers that are missed on multiparametric (mp) MRI are small and “not life-threatening,” according to an analysis of data from the Prostate MR Imaging Study (PROMIS).
“Our work suggests that MRI scans of the prostate appear to deliver crucial information about a man’s risk of dying from prostate cancer, even before he has a biopsy,” said Joseph Norris, BM BS, from University College London.
“This may mean that we can finally move prostate cancer to a position in which we can use imaging as the primary tool to direct further investigations, treatment, and prediction of risk,” he told Medscape Medical News.
This is “a position that all other solid organ cancers have reached,” said Norris, who will present the findings at the upcoming virtual European Association of Urology 2020 Congress.
All 576 PROMIS participants underwent an mpMRI scan, a transrectal ultrasonography (TRUS)–guided biopsy, and a template prostate mapping (TPM) biopsy taken at 5-mm intervals across the entire prostate.
PROMIS researchers previously showed that mpMRI had a 93% sensitivity for clinically significant cancer, whereas TRUS biopsy had only a 48% sensitivity, as reported by Medscape Medical News. And they concluded that the use of mpMRI as a first-line diagnostic tool could prevent 27% of all biopsies, which can have serious adverse effects, such as pain, urinary problems, infection, bleeding, and erectile dysfunction.
However, in their study looking at the accuracy of mpMRI and TRUS biopsy, the researchers did not investigate the severity of the 7% of cancers that mpMRI missed. “What if those missed cancers are, in fact, aggressive? That’s what we set out to examine,” Norris explained.
So he and his colleagues conducted a post ad hoc analysis of the PROMIS participants in whom clinically significant cancer had been detected with TPM biopsy to see which of those cancers had been detected with mpMRI. The findings were published online in European Urology.
Cancers met the strict definition of clinically significant if they had a Gleason score of at least 4+3 for a tumor of any length, or a maximum cancer core length (MCCL) greater than 6 mm for a cancer of any grade. They met the less-strict definition if they had a Gleason score of at least 3+4 for a tumor of any length, or a MCCL greater than 4 mm for a cancer of any grade.
In PROMIS, TPM biopsy detected 230 cancers that met the strict definition of clinically significant and 331 that met the less-strict definition.
Overall Gleason scores were significantly lower for the 17 strict-definition cancers not detected with 1.5 T mpMRI than for those detected with mpMRI (P = .0007), as were maximum Gleason scores (P < .0001).
Median MCCL was 3 mm shorter for all 17 tumors missed with mpMRI than for those detected with mpMRI (5 vs 8 mm; P < .0001).
mpMRI detected all tumors identified on TPM biopsy that had an overall Gleason score greater than 3+4 (Gleason grades 3 to 5) or a maximum Gleason score greater than 4+3 (Gleason grades 4 and 5).
“This finding is important, given that in PROMIS, no men with an overall Gleason score of 4+3 had cancer missed by MRI, indicating that actually MRI may be able to identify all truly significant cancers,” said Norris.
Adding PSA Density Threshold
To further assess cancers missed on mpMRI, the researchers looked at prostate-specific antigen (PSA) density, calculated as total PSA level (ng/mL) divided by prostate volume (mL).
“We found that if we applied a threshold PSA density to men with normal-looking MRI scans, we could reduce the proportion of missed significant cancer to just 5%. This is exciting; it means we can make MRI an even more effective test for prostate cancer in a very simple way,” Norris reported.
“These data show that no highly aggressive prostate cancers were missed by MRI, either at the level of the whole prostate or at the individual needle level,” said Norris. This should lead to positive outcomes in the long term.
And since the PROMIS data were gathered, MRI technology has improved, he said. The “MRI scanners in PROMIS were 1.5 Tesla,” whereas today’s machines are 3.0 T, which could increase the detection of significant prostate cancer.
In fact, “our analysis here potentially overestimates the amount of undetected disease,” he noted.
Prostate cancer that is not clinically significant is often monitored with active surveillance, so “invisible” cancers missed on mpMRI could actually be looked at in a positive light, he explained.
Variation in technique, interpretation
But the quality of care when it comes to the diagnosis of prostate cancer is not equal everywhere, said Gerald Andriole, MD, from the Washington School of Medicine in St. Louis, Missouri.
“When you get an MRI in a center that doesn’t do a lot of prostate cancer testing, you may not have the best software and you may have a radiologist who is not that experienced,” he told Medscape Medical News. Specialized cancer centers of excellence tend to do a great job finding prostate cancer, “but other centers have high significant-miss rates or high overcall rates.”
“The elephant in the room remains the considerable variation in technique and interobserver interpretation of prostate mpMRI,” write Steven Monda, MD, and Marc Dall’Era, MD, both from UC Davis Health in Sacramento, California, in an editorial that accompanies the new PROMIS analysis.
“These problems must be addressed and remedied in each institution before relying on results from PROMIS to drive changes in clinical practice,” they add.
Norris, Andriole, Monda, and Dall’Era have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Low-dose CT lung cancer screening still debated, despite evidence
Despite mounting evidence that low-dose CT screening reduces lung cancer mortality in people at high risk, the uptake of screening in the United States has been slow, and some researchers caution that the risks involved need to be better understood.
It has been almost 10 years since the landmark National Lung Screening Trial (NLST) provided the scientific evidence used by the United States Preventive Services Task Force to recommend annual screening for adults 55 to 80 years of age who have a 30 pack-year smoking history and currently smoke or have quit in the previous 15 years.
But just 4.2% of Americans who qualified for screening in 2018 were tested, according to an American Lung Association report. If everyone at high risk had been tested, 48,000 American lives could have been saved.
Final results from the NELSON trial, published earlier this year, support those from NLST.
Mortality was 24% lower with low-dose CT screening than with no screening in the NELSON cohort, which consisted of 13,195 men and 2594 women at high risk for lung cancer because they were current or former smokers.
“With the NELSON results, the efficacy of low-dose CT screening for lung cancer is confirmed,” wrote the authors of an editorial accompanying the NELSON results. “Our job is no longer to assess whether low-dose CT screening for lung cancer works: it does. Our job is to identify the target population in which it will be acceptable and cost-effective.”
That sentiment is echoed by Michael Gould, MD, from Kaiser Permanente Southern California.
“Lo and behold, we have confirmation of NLST results from NELSON,” Dr. Gould said in an interview. “Now that we have consistent data from the NELSON confirmatory trial, can we finally believe NLST?”
Even though NELSON confirms the benefits of screening in clinical trials, many questions remain about how lung cancer screening translates into everyday practice, said Dr. Gould, who had been scheduled to discuss the trials and the state of lung screening at the American Thoracic Society 2020 International Conference, which will now run virtually in August.
For starters, the target population needs more scrutiny. Research has shown that, outside of clinical trials, the harms of screening can sometimes outweigh the benefits.
In 2018, the rate of overdiagnosis was shown to be 67.2% in the Danish Lung Cancer Screening Trial (DLCST).
And 56% of people screened with low-dose CT had false-positive results that required follow-up testing and procedures, according to a 2017 study of current and former heavy smokers. That rate is more than double the 18.5% false-positive rate in NLST.
“Only 20% of NLST participants were over age 65,” Dr. Gould said. “The NELSON cohort was younger.”
And although the USPSTF recommends lung screening in high-risk people, “there were some in the NLST cohort whose risk was not particularly high.” Others in the trial, he said, had a high risk, but some of those had one or more comorbid conditions, “so the risk was unbalanced.
“Risk is more complicated than simply saying that anyone who meets the NLST criteria should get scanned,” he added.
Weighing risks and benefits needs to be done on a patient-by-patient basis, Dr. Gould said. “Do they have the ability to tolerate surgery? What’s important to them? We can’t just say, ‘you have a 30-pack-year history, go get a test’.”
Often, he said, it’s the people who have the most to gain from screening who are also at highest risk from biopsies and surgical and nonsurgical treatments because of comorbidities.
The NLST population might also have cast a wider net for those eligible for screening; NELSON had a lower threshold for amount smoked (30 vs. 15 pack-years). “NLST points to scanning a bigger population and lighter smokers,” Dr. Gould said.
Psychological risks of screening
Neither the NLST nor NELSON reported relevant psychological aspects of harm from CT screening for lung cancer, two researchers reported in a letter responding to the NELSON findings.
The trial-participation request letters, which were sent to 606,409 people in the general population, “in order to identify 15,792 persons (2.6%) who were eligible to participate, may have caused fear,” wrote Jes Lindholt, MD, DMSc, and Rikke Søgaard, PhD, from Odense University Hospital in Denmark.
“That raises the question: Do people want to be screened? I can’t understand why the US and Britain consider it so definitive to start a screening program,” Dr. Lindholt said in an interview.
In addition to a psychological cost, he questioned the financial cost-benefit ratio of a screening program. “What strikes me is that they haven’t done any cost analysis on any of these randomized trials.”
“Of the 203 men who got the diagnosis of lung cancer, 160 (78.8%) died from lung cancer. Whether screening actually improved or prolonged their remaining lifetime should be considered,” Dr. Lindholt and Dr. Søgaard wrote.
Challenges of implementation
Despite the extensive trials, there are still questions about how to implement screening in the real world. “Did NLST select patients who were, on average, healthier and less likely to have complications?” Dr. Gould asked.
Everyday practice might not find the same favorable outcomes as NLST. “Can the results of the NTLST be replicated in real-world settings? Not yet,” he said. Hospitals and health systems are struggling to implement screening.
Follow-up and tracking are not where they should be. General practitioners don’t have the same resources as the NLST researchers had, he explained. They were able to remind patients to come back for another test and call them with the results, all under the umbrella of implementation, “and they’re still not on target.”
Getting people scanned is key, said Michael Barry, MD, from Massachusetts General Hospital in Boston, who is a current member of the USPSTF and is working on new lung cancer screening recommendations to be published this summer.
“We have an implementation problem,” he said. “The heavier smokers are being way underscreened.”
People need to have more information to review the pros and cons of screening, Dr. Barry said. “We’ve got large trials that show that benefits outweigh the harms, but we could benefit from implementation research. This is an issue for many screening tasks.”
Eight million Americans meet the eligibility requirements for lung cancer screening with low-dose CT, according to a 2019 report from the American College of Radiology.
Screening tests are covered by Medicare, but getting people to the clinic has not been easy. In 2018, Saved by the Scan, a big-budget national advertising campaign launched by the ALA, featured ex-smokers who survived lung cancer because of early detection with a low-dose CT scan, as reported by Medscape Medical News.
And many people being scanned are not part of the USPSTF target group. In 2017, lung cancer screening was reported “by 12.5% of smokers who met USPSTF criteria and 7.9% of smokers aged 55-80 years who did not meet USPSTF criteria,” according to a recent analysis of data from the Behavioral Risk Factor Surveillance System published by the Centers for Disease Control and Prevention.
The CDC report concludes that some people are being screened without needing screening, and that “avoidance of screening inconsistent with USPSTF criteria could reduce the potential for harms such as overdiagnosis and overtreatment.”
Dr. Gould said he agrees that this factor needs to be looked at. “There is underutilization in those who need screening, and maybe overscreening in those who aren’t at risk.”
There are also epidemiologic data that show that black Americans are at higher risk at a younger age for the same level of smoking. “So should there be a lower threshold for smoking and lower age, particularly in the African American population?” Dr. Gould asked.
The NELSON trial had significant results in a population younger than that in NLST, he pointed out. “That needs to be considered.”
Smokers dismiss medical advice
People in the high-risk group need to better understand the benefits of screening, said Christine D. Berg, MD, an NLST researcher from the National Cancer Institute.
“We know the uptake of lung cancer screening has been slow,” she said.
She described encouraging her neighbor, a heavy smoker, to get screened. “But she said she didn’t want to know if she had lung cancer, so she didn’t go.”
“Now she’s dead,” Dr. Berg continued. Unfortunately, “what we see is that those who continue to smoke, and smoke heavily, are not likely to heed medical advice.”
The fear of finding out you have lung cancer needs to be overcome, she said. Smokers need to understand that they can add a decade to their lives if lung cancer is detected early.
Some places in the United States have better screening rates than others. “We see a lot of variation from state to state,” she said. For instance, in Massachusetts, 12.3% of high-risk people have been screened; in Nevada, the rate is just 0.5%.
There are many reasons for that. First, there are logistics. Screening covered by Medicare must be done in a certified center “with good equipment and that can track results,” Dr. Berg said. That might be one hurdle. But the greater hurdle is the patients themselves.
There are studies that point to risks associated with invasive procedures, such as biopsy after screening, which can lead to complications, even when no cancer is found. “My answer to that is, if you need a biopsy, check the data. The Society of Thoracic Surgeons has a database of all the complications, and it’s publicly accessible. You can find hospitals in your region that report data,” she explained, and “that have highest volume and lowest complication rates.”
Second, imaging has improved since the NLST trial. “We have a better ability to estimate cancer in the nodules we find,” Dr. Berg explained. Nodules that previously needed a biopsy to confirm malignancy can now be assessed with AI and machine learning.
“I think the probability of false positives and problems from biopsy have changed dramatically over the last 10 years,” she said.
And we are catching more lung cancer earlier and saving lives. Overall, early detection is increasing, and late-stage detection is decreasing. “We’re bending the curve, making progress,” she said.
In 2019, the 5-year survival rate for lung cancer was 21.7%, up from 17.2% a decade earlier, according to the ALA. Much of that is because of early diagnosis, when the disease is still curable, which could be related to increased screening.
“NELSON showed benefit to CT screening and is useful in helping convince some of the skeptics,” Dr. Berg said.
Diagnosis is also improving with new technologies. Electronic health records can be scanned to identify patients at increased risk, and patient portals can send reminders, notifications, and other educational information to encourage patients to discuss options with their doctor, which could improve the national lung cancer prognosis, Dr. Gould said.
At the end of the day, it still comes down to the patient and doctor having a conversation about the risks and benefits.
“But we have to get to that point,” Dr. Gould said. “We need to continue to develop tools to facilitate that conversation. It’s complicated, and there’s a lot of information to weigh.”
“We’re still working out how to do that,” he added.
Dr. Barry, Dr. Gould, and Dr. Berg have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Despite mounting evidence that low-dose CT screening reduces lung cancer mortality in people at high risk, the uptake of screening in the United States has been slow, and some researchers caution that the risks involved need to be better understood.
It has been almost 10 years since the landmark National Lung Screening Trial (NLST) provided the scientific evidence used by the United States Preventive Services Task Force to recommend annual screening for adults 55 to 80 years of age who have a 30 pack-year smoking history and currently smoke or have quit in the previous 15 years.
But just 4.2% of Americans who qualified for screening in 2018 were tested, according to an American Lung Association report. If everyone at high risk had been tested, 48,000 American lives could have been saved.
Final results from the NELSON trial, published earlier this year, support those from NLST.
Mortality was 24% lower with low-dose CT screening than with no screening in the NELSON cohort, which consisted of 13,195 men and 2594 women at high risk for lung cancer because they were current or former smokers.
“With the NELSON results, the efficacy of low-dose CT screening for lung cancer is confirmed,” wrote the authors of an editorial accompanying the NELSON results. “Our job is no longer to assess whether low-dose CT screening for lung cancer works: it does. Our job is to identify the target population in which it will be acceptable and cost-effective.”
That sentiment is echoed by Michael Gould, MD, from Kaiser Permanente Southern California.
“Lo and behold, we have confirmation of NLST results from NELSON,” Dr. Gould said in an interview. “Now that we have consistent data from the NELSON confirmatory trial, can we finally believe NLST?”
Even though NELSON confirms the benefits of screening in clinical trials, many questions remain about how lung cancer screening translates into everyday practice, said Dr. Gould, who had been scheduled to discuss the trials and the state of lung screening at the American Thoracic Society 2020 International Conference, which will now run virtually in August.
For starters, the target population needs more scrutiny. Research has shown that, outside of clinical trials, the harms of screening can sometimes outweigh the benefits.
In 2018, the rate of overdiagnosis was shown to be 67.2% in the Danish Lung Cancer Screening Trial (DLCST).
And 56% of people screened with low-dose CT had false-positive results that required follow-up testing and procedures, according to a 2017 study of current and former heavy smokers. That rate is more than double the 18.5% false-positive rate in NLST.
“Only 20% of NLST participants were over age 65,” Dr. Gould said. “The NELSON cohort was younger.”
And although the USPSTF recommends lung screening in high-risk people, “there were some in the NLST cohort whose risk was not particularly high.” Others in the trial, he said, had a high risk, but some of those had one or more comorbid conditions, “so the risk was unbalanced.
“Risk is more complicated than simply saying that anyone who meets the NLST criteria should get scanned,” he added.
Weighing risks and benefits needs to be done on a patient-by-patient basis, Dr. Gould said. “Do they have the ability to tolerate surgery? What’s important to them? We can’t just say, ‘you have a 30-pack-year history, go get a test’.”
Often, he said, it’s the people who have the most to gain from screening who are also at highest risk from biopsies and surgical and nonsurgical treatments because of comorbidities.
The NLST population might also have cast a wider net for those eligible for screening; NELSON had a lower threshold for amount smoked (30 vs. 15 pack-years). “NLST points to scanning a bigger population and lighter smokers,” Dr. Gould said.
Psychological risks of screening
Neither the NLST nor NELSON reported relevant psychological aspects of harm from CT screening for lung cancer, two researchers reported in a letter responding to the NELSON findings.
The trial-participation request letters, which were sent to 606,409 people in the general population, “in order to identify 15,792 persons (2.6%) who were eligible to participate, may have caused fear,” wrote Jes Lindholt, MD, DMSc, and Rikke Søgaard, PhD, from Odense University Hospital in Denmark.
“That raises the question: Do people want to be screened? I can’t understand why the US and Britain consider it so definitive to start a screening program,” Dr. Lindholt said in an interview.
In addition to a psychological cost, he questioned the financial cost-benefit ratio of a screening program. “What strikes me is that they haven’t done any cost analysis on any of these randomized trials.”
“Of the 203 men who got the diagnosis of lung cancer, 160 (78.8%) died from lung cancer. Whether screening actually improved or prolonged their remaining lifetime should be considered,” Dr. Lindholt and Dr. Søgaard wrote.
Challenges of implementation
Despite the extensive trials, there are still questions about how to implement screening in the real world. “Did NLST select patients who were, on average, healthier and less likely to have complications?” Dr. Gould asked.
Everyday practice might not find the same favorable outcomes as NLST. “Can the results of the NTLST be replicated in real-world settings? Not yet,” he said. Hospitals and health systems are struggling to implement screening.
Follow-up and tracking are not where they should be. General practitioners don’t have the same resources as the NLST researchers had, he explained. They were able to remind patients to come back for another test and call them with the results, all under the umbrella of implementation, “and they’re still not on target.”
Getting people scanned is key, said Michael Barry, MD, from Massachusetts General Hospital in Boston, who is a current member of the USPSTF and is working on new lung cancer screening recommendations to be published this summer.
“We have an implementation problem,” he said. “The heavier smokers are being way underscreened.”
People need to have more information to review the pros and cons of screening, Dr. Barry said. “We’ve got large trials that show that benefits outweigh the harms, but we could benefit from implementation research. This is an issue for many screening tasks.”
Eight million Americans meet the eligibility requirements for lung cancer screening with low-dose CT, according to a 2019 report from the American College of Radiology.
Screening tests are covered by Medicare, but getting people to the clinic has not been easy. In 2018, Saved by the Scan, a big-budget national advertising campaign launched by the ALA, featured ex-smokers who survived lung cancer because of early detection with a low-dose CT scan, as reported by Medscape Medical News.
And many people being scanned are not part of the USPSTF target group. In 2017, lung cancer screening was reported “by 12.5% of smokers who met USPSTF criteria and 7.9% of smokers aged 55-80 years who did not meet USPSTF criteria,” according to a recent analysis of data from the Behavioral Risk Factor Surveillance System published by the Centers for Disease Control and Prevention.
The CDC report concludes that some people are being screened without needing screening, and that “avoidance of screening inconsistent with USPSTF criteria could reduce the potential for harms such as overdiagnosis and overtreatment.”
Dr. Gould said he agrees that this factor needs to be looked at. “There is underutilization in those who need screening, and maybe overscreening in those who aren’t at risk.”
There are also epidemiologic data that show that black Americans are at higher risk at a younger age for the same level of smoking. “So should there be a lower threshold for smoking and lower age, particularly in the African American population?” Dr. Gould asked.
The NELSON trial had significant results in a population younger than that in NLST, he pointed out. “That needs to be considered.”
Smokers dismiss medical advice
People in the high-risk group need to better understand the benefits of screening, said Christine D. Berg, MD, an NLST researcher from the National Cancer Institute.
“We know the uptake of lung cancer screening has been slow,” she said.
She described encouraging her neighbor, a heavy smoker, to get screened. “But she said she didn’t want to know if she had lung cancer, so she didn’t go.”
“Now she’s dead,” Dr. Berg continued. Unfortunately, “what we see is that those who continue to smoke, and smoke heavily, are not likely to heed medical advice.”
The fear of finding out you have lung cancer needs to be overcome, she said. Smokers need to understand that they can add a decade to their lives if lung cancer is detected early.
Some places in the United States have better screening rates than others. “We see a lot of variation from state to state,” she said. For instance, in Massachusetts, 12.3% of high-risk people have been screened; in Nevada, the rate is just 0.5%.
There are many reasons for that. First, there are logistics. Screening covered by Medicare must be done in a certified center “with good equipment and that can track results,” Dr. Berg said. That might be one hurdle. But the greater hurdle is the patients themselves.
There are studies that point to risks associated with invasive procedures, such as biopsy after screening, which can lead to complications, even when no cancer is found. “My answer to that is, if you need a biopsy, check the data. The Society of Thoracic Surgeons has a database of all the complications, and it’s publicly accessible. You can find hospitals in your region that report data,” she explained, and “that have highest volume and lowest complication rates.”
Second, imaging has improved since the NLST trial. “We have a better ability to estimate cancer in the nodules we find,” Dr. Berg explained. Nodules that previously needed a biopsy to confirm malignancy can now be assessed with AI and machine learning.
“I think the probability of false positives and problems from biopsy have changed dramatically over the last 10 years,” she said.
And we are catching more lung cancer earlier and saving lives. Overall, early detection is increasing, and late-stage detection is decreasing. “We’re bending the curve, making progress,” she said.
In 2019, the 5-year survival rate for lung cancer was 21.7%, up from 17.2% a decade earlier, according to the ALA. Much of that is because of early diagnosis, when the disease is still curable, which could be related to increased screening.
“NELSON showed benefit to CT screening and is useful in helping convince some of the skeptics,” Dr. Berg said.
Diagnosis is also improving with new technologies. Electronic health records can be scanned to identify patients at increased risk, and patient portals can send reminders, notifications, and other educational information to encourage patients to discuss options with their doctor, which could improve the national lung cancer prognosis, Dr. Gould said.
At the end of the day, it still comes down to the patient and doctor having a conversation about the risks and benefits.
“But we have to get to that point,” Dr. Gould said. “We need to continue to develop tools to facilitate that conversation. It’s complicated, and there’s a lot of information to weigh.”
“We’re still working out how to do that,” he added.
Dr. Barry, Dr. Gould, and Dr. Berg have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Despite mounting evidence that low-dose CT screening reduces lung cancer mortality in people at high risk, the uptake of screening in the United States has been slow, and some researchers caution that the risks involved need to be better understood.
It has been almost 10 years since the landmark National Lung Screening Trial (NLST) provided the scientific evidence used by the United States Preventive Services Task Force to recommend annual screening for adults 55 to 80 years of age who have a 30 pack-year smoking history and currently smoke or have quit in the previous 15 years.
But just 4.2% of Americans who qualified for screening in 2018 were tested, according to an American Lung Association report. If everyone at high risk had been tested, 48,000 American lives could have been saved.
Final results from the NELSON trial, published earlier this year, support those from NLST.
Mortality was 24% lower with low-dose CT screening than with no screening in the NELSON cohort, which consisted of 13,195 men and 2594 women at high risk for lung cancer because they were current or former smokers.
“With the NELSON results, the efficacy of low-dose CT screening for lung cancer is confirmed,” wrote the authors of an editorial accompanying the NELSON results. “Our job is no longer to assess whether low-dose CT screening for lung cancer works: it does. Our job is to identify the target population in which it will be acceptable and cost-effective.”
That sentiment is echoed by Michael Gould, MD, from Kaiser Permanente Southern California.
“Lo and behold, we have confirmation of NLST results from NELSON,” Dr. Gould said in an interview. “Now that we have consistent data from the NELSON confirmatory trial, can we finally believe NLST?”
Even though NELSON confirms the benefits of screening in clinical trials, many questions remain about how lung cancer screening translates into everyday practice, said Dr. Gould, who had been scheduled to discuss the trials and the state of lung screening at the American Thoracic Society 2020 International Conference, which will now run virtually in August.
For starters, the target population needs more scrutiny. Research has shown that, outside of clinical trials, the harms of screening can sometimes outweigh the benefits.
In 2018, the rate of overdiagnosis was shown to be 67.2% in the Danish Lung Cancer Screening Trial (DLCST).
And 56% of people screened with low-dose CT had false-positive results that required follow-up testing and procedures, according to a 2017 study of current and former heavy smokers. That rate is more than double the 18.5% false-positive rate in NLST.
“Only 20% of NLST participants were over age 65,” Dr. Gould said. “The NELSON cohort was younger.”
And although the USPSTF recommends lung screening in high-risk people, “there were some in the NLST cohort whose risk was not particularly high.” Others in the trial, he said, had a high risk, but some of those had one or more comorbid conditions, “so the risk was unbalanced.
“Risk is more complicated than simply saying that anyone who meets the NLST criteria should get scanned,” he added.
Weighing risks and benefits needs to be done on a patient-by-patient basis, Dr. Gould said. “Do they have the ability to tolerate surgery? What’s important to them? We can’t just say, ‘you have a 30-pack-year history, go get a test’.”
Often, he said, it’s the people who have the most to gain from screening who are also at highest risk from biopsies and surgical and nonsurgical treatments because of comorbidities.
The NLST population might also have cast a wider net for those eligible for screening; NELSON had a lower threshold for amount smoked (30 vs. 15 pack-years). “NLST points to scanning a bigger population and lighter smokers,” Dr. Gould said.
Psychological risks of screening
Neither the NLST nor NELSON reported relevant psychological aspects of harm from CT screening for lung cancer, two researchers reported in a letter responding to the NELSON findings.
The trial-participation request letters, which were sent to 606,409 people in the general population, “in order to identify 15,792 persons (2.6%) who were eligible to participate, may have caused fear,” wrote Jes Lindholt, MD, DMSc, and Rikke Søgaard, PhD, from Odense University Hospital in Denmark.
“That raises the question: Do people want to be screened? I can’t understand why the US and Britain consider it so definitive to start a screening program,” Dr. Lindholt said in an interview.
In addition to a psychological cost, he questioned the financial cost-benefit ratio of a screening program. “What strikes me is that they haven’t done any cost analysis on any of these randomized trials.”
“Of the 203 men who got the diagnosis of lung cancer, 160 (78.8%) died from lung cancer. Whether screening actually improved or prolonged their remaining lifetime should be considered,” Dr. Lindholt and Dr. Søgaard wrote.
Challenges of implementation
Despite the extensive trials, there are still questions about how to implement screening in the real world. “Did NLST select patients who were, on average, healthier and less likely to have complications?” Dr. Gould asked.
Everyday practice might not find the same favorable outcomes as NLST. “Can the results of the NTLST be replicated in real-world settings? Not yet,” he said. Hospitals and health systems are struggling to implement screening.
Follow-up and tracking are not where they should be. General practitioners don’t have the same resources as the NLST researchers had, he explained. They were able to remind patients to come back for another test and call them with the results, all under the umbrella of implementation, “and they’re still not on target.”
Getting people scanned is key, said Michael Barry, MD, from Massachusetts General Hospital in Boston, who is a current member of the USPSTF and is working on new lung cancer screening recommendations to be published this summer.
“We have an implementation problem,” he said. “The heavier smokers are being way underscreened.”
People need to have more information to review the pros and cons of screening, Dr. Barry said. “We’ve got large trials that show that benefits outweigh the harms, but we could benefit from implementation research. This is an issue for many screening tasks.”
Eight million Americans meet the eligibility requirements for lung cancer screening with low-dose CT, according to a 2019 report from the American College of Radiology.
Screening tests are covered by Medicare, but getting people to the clinic has not been easy. In 2018, Saved by the Scan, a big-budget national advertising campaign launched by the ALA, featured ex-smokers who survived lung cancer because of early detection with a low-dose CT scan, as reported by Medscape Medical News.
And many people being scanned are not part of the USPSTF target group. In 2017, lung cancer screening was reported “by 12.5% of smokers who met USPSTF criteria and 7.9% of smokers aged 55-80 years who did not meet USPSTF criteria,” according to a recent analysis of data from the Behavioral Risk Factor Surveillance System published by the Centers for Disease Control and Prevention.
The CDC report concludes that some people are being screened without needing screening, and that “avoidance of screening inconsistent with USPSTF criteria could reduce the potential for harms such as overdiagnosis and overtreatment.”
Dr. Gould said he agrees that this factor needs to be looked at. “There is underutilization in those who need screening, and maybe overscreening in those who aren’t at risk.”
There are also epidemiologic data that show that black Americans are at higher risk at a younger age for the same level of smoking. “So should there be a lower threshold for smoking and lower age, particularly in the African American population?” Dr. Gould asked.
The NELSON trial had significant results in a population younger than that in NLST, he pointed out. “That needs to be considered.”
Smokers dismiss medical advice
People in the high-risk group need to better understand the benefits of screening, said Christine D. Berg, MD, an NLST researcher from the National Cancer Institute.
“We know the uptake of lung cancer screening has been slow,” she said.
She described encouraging her neighbor, a heavy smoker, to get screened. “But she said she didn’t want to know if she had lung cancer, so she didn’t go.”
“Now she’s dead,” Dr. Berg continued. Unfortunately, “what we see is that those who continue to smoke, and smoke heavily, are not likely to heed medical advice.”
The fear of finding out you have lung cancer needs to be overcome, she said. Smokers need to understand that they can add a decade to their lives if lung cancer is detected early.
Some places in the United States have better screening rates than others. “We see a lot of variation from state to state,” she said. For instance, in Massachusetts, 12.3% of high-risk people have been screened; in Nevada, the rate is just 0.5%.
There are many reasons for that. First, there are logistics. Screening covered by Medicare must be done in a certified center “with good equipment and that can track results,” Dr. Berg said. That might be one hurdle. But the greater hurdle is the patients themselves.
There are studies that point to risks associated with invasive procedures, such as biopsy after screening, which can lead to complications, even when no cancer is found. “My answer to that is, if you need a biopsy, check the data. The Society of Thoracic Surgeons has a database of all the complications, and it’s publicly accessible. You can find hospitals in your region that report data,” she explained, and “that have highest volume and lowest complication rates.”
Second, imaging has improved since the NLST trial. “We have a better ability to estimate cancer in the nodules we find,” Dr. Berg explained. Nodules that previously needed a biopsy to confirm malignancy can now be assessed with AI and machine learning.
“I think the probability of false positives and problems from biopsy have changed dramatically over the last 10 years,” she said.
And we are catching more lung cancer earlier and saving lives. Overall, early detection is increasing, and late-stage detection is decreasing. “We’re bending the curve, making progress,” she said.
In 2019, the 5-year survival rate for lung cancer was 21.7%, up from 17.2% a decade earlier, according to the ALA. Much of that is because of early diagnosis, when the disease is still curable, which could be related to increased screening.
“NELSON showed benefit to CT screening and is useful in helping convince some of the skeptics,” Dr. Berg said.
Diagnosis is also improving with new technologies. Electronic health records can be scanned to identify patients at increased risk, and patient portals can send reminders, notifications, and other educational information to encourage patients to discuss options with their doctor, which could improve the national lung cancer prognosis, Dr. Gould said.
At the end of the day, it still comes down to the patient and doctor having a conversation about the risks and benefits.
“But we have to get to that point,” Dr. Gould said. “We need to continue to develop tools to facilitate that conversation. It’s complicated, and there’s a lot of information to weigh.”
“We’re still working out how to do that,” he added.
Dr. Barry, Dr. Gould, and Dr. Berg have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Smokers who are unmotivated to quit smoke more with e-cigarettes
Not only does the use of e-cigarettes not help cigarette smokers who are unmotivated to quit, it has the opposite effect, according to results from a new study.
“In our study, people vaping in addition to smoking actually started smoking more,” said study lead investigator Nancy Anoruo, MD, from the University of Massachusetts Medical School in Worcester.
This is “completely contradictory to what the e-cigarette manufacturers are telling us,” she told this news organization.
In their study, Dr. Anoruo and her colleagues looked at whether people were more likely to quit if they smoked e-cigarettes in addition to conventional cigarettes. The research is a substudy of the ongoing Take a Break project, funded by the National Institutes of Health, which is assessing whether a smoking-cessation motivation app helps smokers quit.
In a cohort of 405 smokers who were unmotivated to quit, 248 were defined as dual smokers after responding “yes” to “ever having used” e-cigarettes, and 157 were defined as traditional smokers who only smoked combustible cigarettes. The majority of participants, 82%, were white; 8.8% were black; and 49% were women.
More dual smokers than traditional smokers were younger than 40 years (27% vs. 16%; (P = .02), Dr. Anoruo reported during her virtual presentation at the American Thoracic Society 2020 International Conference.
The dual smokers reported smoking an average of 16 cigarettes a day, compared with 14 a day for the traditional smokers.
All the smokers were encouraged to consider a 3-week period of abstinence from combustible cigarettes. At the end of that period, the researchers compared outcomes reported by participants.
Abstinence challenge
Average abstinence intervals were shorter for dual smokers than for traditional smokers (0.93 vs. 1.8 days; P = .01). And dual smokers reported having a harder time quitting completely (6.3% vs. 13.0%; P = .02).
At 6-month follow-up, dual smokers were smoking more cigarettes than traditional smokers (daily average, 12.0 vs. 9.4; P = .04). And the reduction in cigarette use from baseline was smaller for dual smokers than for traditional smokers (21% vs. 33%; P = .04).
“E-cigarettes are not a special magic bullet to get people to quit smoking,” said Dr. Anoruo.
In this study, smoking cessation was defined as abstinence from combustible cigarettes, but that did not mean participants were abstinent from nicotine.
“If, at the end, they stopped smoking traditional cigarettes, we considered that successful smoking cessation,” Dr. Anoruo explained. This definition is in line with the school of thought that e-cigarettes are a harm-reduction tool.
“But we now know that e-cigarettes are not necessarily safe,” she added.
Still, it might be the lesser evil. “You end up taking in less dangerous chemicals, so we consider it quitting if you get off regular cigarettes,” she said.
“We would like to study the psychology of cigarette smokers to find out if they see e-cigarettes as a smoking-cessation aid,” Dr. Anoruo said, and to see if “their belief is driven by the advertising they see about e-cigarette use.”
A meager reduction
Similar results were shown last month in a study by Megan Piper, PhD, of University of Wisconsin–Madison and her colleagues, who reported that dual e-cigarette and combustible cigarette use “did not appear to be an effective path to cessation of combustible cigarettes.”
After 1 year, dual smokers smoked three cigarettes less each day than traditional smokers, which is “a meager reduction,” Dr. Piper said in a news release.
“Typically, you can’t have one foot in both camps. Most can’t be vaping and smoking and hope to quit smoking,” she added. “That sustained pattern is not going to help most people quit.”
A version of this article originally appeared on Medscape.com.
Not only does the use of e-cigarettes not help cigarette smokers who are unmotivated to quit, it has the opposite effect, according to results from a new study.
“In our study, people vaping in addition to smoking actually started smoking more,” said study lead investigator Nancy Anoruo, MD, from the University of Massachusetts Medical School in Worcester.
This is “completely contradictory to what the e-cigarette manufacturers are telling us,” she told this news organization.
In their study, Dr. Anoruo and her colleagues looked at whether people were more likely to quit if they smoked e-cigarettes in addition to conventional cigarettes. The research is a substudy of the ongoing Take a Break project, funded by the National Institutes of Health, which is assessing whether a smoking-cessation motivation app helps smokers quit.
In a cohort of 405 smokers who were unmotivated to quit, 248 were defined as dual smokers after responding “yes” to “ever having used” e-cigarettes, and 157 were defined as traditional smokers who only smoked combustible cigarettes. The majority of participants, 82%, were white; 8.8% were black; and 49% were women.
More dual smokers than traditional smokers were younger than 40 years (27% vs. 16%; (P = .02), Dr. Anoruo reported during her virtual presentation at the American Thoracic Society 2020 International Conference.
The dual smokers reported smoking an average of 16 cigarettes a day, compared with 14 a day for the traditional smokers.
All the smokers were encouraged to consider a 3-week period of abstinence from combustible cigarettes. At the end of that period, the researchers compared outcomes reported by participants.
Abstinence challenge
Average abstinence intervals were shorter for dual smokers than for traditional smokers (0.93 vs. 1.8 days; P = .01). And dual smokers reported having a harder time quitting completely (6.3% vs. 13.0%; P = .02).
At 6-month follow-up, dual smokers were smoking more cigarettes than traditional smokers (daily average, 12.0 vs. 9.4; P = .04). And the reduction in cigarette use from baseline was smaller for dual smokers than for traditional smokers (21% vs. 33%; P = .04).
“E-cigarettes are not a special magic bullet to get people to quit smoking,” said Dr. Anoruo.
In this study, smoking cessation was defined as abstinence from combustible cigarettes, but that did not mean participants were abstinent from nicotine.
“If, at the end, they stopped smoking traditional cigarettes, we considered that successful smoking cessation,” Dr. Anoruo explained. This definition is in line with the school of thought that e-cigarettes are a harm-reduction tool.
“But we now know that e-cigarettes are not necessarily safe,” she added.
Still, it might be the lesser evil. “You end up taking in less dangerous chemicals, so we consider it quitting if you get off regular cigarettes,” she said.
“We would like to study the psychology of cigarette smokers to find out if they see e-cigarettes as a smoking-cessation aid,” Dr. Anoruo said, and to see if “their belief is driven by the advertising they see about e-cigarette use.”
A meager reduction
Similar results were shown last month in a study by Megan Piper, PhD, of University of Wisconsin–Madison and her colleagues, who reported that dual e-cigarette and combustible cigarette use “did not appear to be an effective path to cessation of combustible cigarettes.”
After 1 year, dual smokers smoked three cigarettes less each day than traditional smokers, which is “a meager reduction,” Dr. Piper said in a news release.
“Typically, you can’t have one foot in both camps. Most can’t be vaping and smoking and hope to quit smoking,” she added. “That sustained pattern is not going to help most people quit.”
A version of this article originally appeared on Medscape.com.
Not only does the use of e-cigarettes not help cigarette smokers who are unmotivated to quit, it has the opposite effect, according to results from a new study.
“In our study, people vaping in addition to smoking actually started smoking more,” said study lead investigator Nancy Anoruo, MD, from the University of Massachusetts Medical School in Worcester.
This is “completely contradictory to what the e-cigarette manufacturers are telling us,” she told this news organization.
In their study, Dr. Anoruo and her colleagues looked at whether people were more likely to quit if they smoked e-cigarettes in addition to conventional cigarettes. The research is a substudy of the ongoing Take a Break project, funded by the National Institutes of Health, which is assessing whether a smoking-cessation motivation app helps smokers quit.
In a cohort of 405 smokers who were unmotivated to quit, 248 were defined as dual smokers after responding “yes” to “ever having used” e-cigarettes, and 157 were defined as traditional smokers who only smoked combustible cigarettes. The majority of participants, 82%, were white; 8.8% were black; and 49% were women.
More dual smokers than traditional smokers were younger than 40 years (27% vs. 16%; (P = .02), Dr. Anoruo reported during her virtual presentation at the American Thoracic Society 2020 International Conference.
The dual smokers reported smoking an average of 16 cigarettes a day, compared with 14 a day for the traditional smokers.
All the smokers were encouraged to consider a 3-week period of abstinence from combustible cigarettes. At the end of that period, the researchers compared outcomes reported by participants.
Abstinence challenge
Average abstinence intervals were shorter for dual smokers than for traditional smokers (0.93 vs. 1.8 days; P = .01). And dual smokers reported having a harder time quitting completely (6.3% vs. 13.0%; P = .02).
At 6-month follow-up, dual smokers were smoking more cigarettes than traditional smokers (daily average, 12.0 vs. 9.4; P = .04). And the reduction in cigarette use from baseline was smaller for dual smokers than for traditional smokers (21% vs. 33%; P = .04).
“E-cigarettes are not a special magic bullet to get people to quit smoking,” said Dr. Anoruo.
In this study, smoking cessation was defined as abstinence from combustible cigarettes, but that did not mean participants were abstinent from nicotine.
“If, at the end, they stopped smoking traditional cigarettes, we considered that successful smoking cessation,” Dr. Anoruo explained. This definition is in line with the school of thought that e-cigarettes are a harm-reduction tool.
“But we now know that e-cigarettes are not necessarily safe,” she added.
Still, it might be the lesser evil. “You end up taking in less dangerous chemicals, so we consider it quitting if you get off regular cigarettes,” she said.
“We would like to study the psychology of cigarette smokers to find out if they see e-cigarettes as a smoking-cessation aid,” Dr. Anoruo said, and to see if “their belief is driven by the advertising they see about e-cigarette use.”
A meager reduction
Similar results were shown last month in a study by Megan Piper, PhD, of University of Wisconsin–Madison and her colleagues, who reported that dual e-cigarette and combustible cigarette use “did not appear to be an effective path to cessation of combustible cigarettes.”
After 1 year, dual smokers smoked three cigarettes less each day than traditional smokers, which is “a meager reduction,” Dr. Piper said in a news release.
“Typically, you can’t have one foot in both camps. Most can’t be vaping and smoking and hope to quit smoking,” she added. “That sustained pattern is not going to help most people quit.”
A version of this article originally appeared on Medscape.com.